Larger image (546 kB)
Title: A Practical Treatise on Gas-light
Author: Friedrich Christian Accum
Release date: January 2, 2014 [eBook #44567]
Language: English
Credits: Produced by Chris Curnow, Harry Lamé and the Online
Distributed Proofreading Team at http://www.pgdp.net (This
file was produced from images generously made available
by The Internet Archive)
Please see the Transcriber’s Notes at the end of this text.
By FREDRICK ACCUM,
OPERATIVE CHEMIST,
LECTURER ON PRACTICAL CHEMISTRY, ON MINERALOGY, AND ON CHEMISTRY
APPLIED TO THE ARTS AND MANUFACTURES; MEMBER OF THE ROYAL
IRISH ACADEMY, FELLOW OF THE LINNÆN SOCIETY, MEMBER
OF THE ROYAL ACADEMY OF SCIENCES OF BERLIN, &c. &c.
WITH SEVEN COLOURED PLATES.
London:
PRINTED BY G. HAYDEN, BRYDGES-STREET, COVENT GARDEN;
FOR R. ACKERMANN, 101, STRAND;
LONGMAN, HURST, REES, ORME, AND BROWN; AND SHERWOOD, NEELY, AND
JONES, PATERNOSTER ROW; AND J. HATCHARD, PICCADILLY.
Price—Twelve Shillings in Boards.
1815.
EX FUMO DARE LUCEM.
Hor.
11, Compton Street Soho.
The following pages are intended to exhibit a summary view of the new art of procuring light, by means of carburetted hydrogen gas obtained from pit-coal, and which of late has been employed with unparalelled success, as a substitute for candles and lamps, and is known by the name of Gas-Light.
To accomplish this object, I have given, in the first part of this Essay, a concise and popular view of the chemical theory and production of artificial light—I have explained the action of candles and lamps—I have shown the methods of measuring the comparative illuminating power of artificial light of different kinds, so as to appreciate their economical value—I have stated the proportions of combustible materials requisite for producing a light of a certain strength; with such other preliminary facts and observations as were deemed necessary to enable the reader to understand fully the nature of the new art of illumination, which it is the object of this Essay to describe.
These positions are followed by a chemical view of the general nature and composition of coal—the chemical changes which this substance suffers, when employed in the production of gas-light—the different[ii] products it furnishes—the modes of obtaining them—their properties and applications in the various arts of life.
I have given a description of the apparatus and machinery by means of which the coal-gas is prepared, and the methods employed for distributing and applying it as a substitute for candles and lamps to illuminate houses, streets and manufactories;—I have furnished the data for calculating the expense that must attend the application of this species of light under different circumstances, so as to determine the relative cost or value of gas-lights, when compared with the lights now in use—together with such other practical directions and facts as will enable the reader to form a proper estimate of the gas-light illumination, and to put this art into practice.
I have stated the leading objects of public and private utility to which the new system of lighting may be successfully applied, candidly pointing out those in which it cannot be made use of to advantage.
I have detailed the most obvious effects which the discovery of lighting with coal-gas must inevitably produce upon the arts and upon domestic economy; its primary advantages—its views—its limits, and the resources it presents to industry and public economy. I have endeavoured to show how far its application is safe, and in what respect it is entitled to public approbation and national encouragement.
It may not be improper, before concluding, to inform the reader, that my qualifications for the task I have undertaken are founded upon many years experience, during which time, I possessed peculiar opportunities to witness and verify the most extended series of operations that ever have been made for the purpose of ascertaining the practicability, safety, and general nature of the art of applying coal-gas as a substitute for tallow and oil; and which have, as it were, fixed the fate of this art. The numerous experiments I instituted, upon a large scale, by desire of the Gas-Light Company, for the purpose of adducing them in my evidence before the House of Commons, and House of Lords, on a former occasion, have enabled me to collect such information as could not have been obtained by other means. The substance of these results (which are printed by order of Government,) are incorporated in this Treatise, together with such other facts and observations as have presented themselves in the routine of my profession elsewhere.
To generalize the results of my observations, and to make them practically useful to the public, is the aim of the present publication, and I need scarcely add, that their suffrages to the zeal and industry, at least, with which I have endeavoured to attain my object, will be a source of infinite satisfaction.
FREDRICK ACCUM
| INTRODUCTORY OBSERVATION. | Page | 1. |
| Progress of the arts.—Influence of it upon the morals and condition of man.—Beneficial tendency of chemical and mechanical improvements.—State of pre-eminence of people with regard to civilization.—How to be estimated.—Flourishing state of those nations which have shown the greatest activity in cultivating the useful arts, and establishing useful enterprises.—General observations on this subject.—Extraordinary discoveries of modern times.—New art of procuring light.—Object of the treatise. | ||
| PART I. | ||
| PRODUCTION OF ARTIFICIAL LIGHT, &c. | 8. | |
| Production of the flame generated during the combustion of certain bodies.—Characters of flame when perfect.—Most luminous flame, how produced with the least consumption of combustible matter.—Conditions necessary for that purpose.—Importance of this subject, with regard to the production and supply of artificial light.—The flame of bodies may be tinged.—Blue flame, red flame, green flame, &c.—Opinion concerning the origin of light emitted by bodies burning with flame.—Philosophy of the subject.—Theory of the action of the instruments of illumination.—Rude method of procuring light employed in some countries.—Chemical action of candles, and lamps.—Agency of the tallow, oil, &c.—Office of the wick.—Reason why tallow candles require snuffing, and wax candles snuff themselves—Further observations on the subject. | ||
| METHOD OF ASCERTAINING THE ILLUMINATING POWER OF CANDLES, LAMPS, AND OTHER LUMINOUS BODIES. | 22. | |
| Optical principle assumed as law for determining the relative strength of lights of different kinds.—Admeasurement of the intensities of light.—Quantity of wax, tallow, oil, &c. requisite for producing a light of a certain strength.—Method of increasing the light of tallow candles, and to obviate the necessity of snuffing them.—A tallow candle placed in an inclined position gives more light than when placed perpendicularly and snuffed with an instrument.—Explanation of the fact.—Further observations on this subject.—Comparative cost of the light obtained by burning tallow candles of different sorts and sizes. | ||
| PART II.[ii] | ||
| GAS-LIGHT. | 47. | |
| Encouragement given by the legislature to the new system of procuring light.—Gas-light company, incorporated by charter, to apply the new art of illumination by way of experiment, on a large scale, to illuminate the streets and houses of the metropolis.—Power and authorities granted to this corporate body.—are very restricted, and do not prevent other individuals from entering into competition with them.—Boundaries of their experiments.—limit of capital employed by them.—Power of His Majesty with regard to the gas-light charter. | ||
| THEORY OF THE COMBUSTION OF COAL IN ELUCIDATION OF THE NATURE OF GAS-LIGHT. | 49. | |
| Natural history of pit-coal.—Immediate constituent parts of coal.—Their relative quantities—are different in different kinds of coal.—Phenomena, which happen during the combustion of coal.—Analysis of coal by distillation.—Great waste of matter capable of producing light and heat, in the usual mode of burning coal.—Proofs of this statement.—Theory of the production of gas-light, compared with the production of light obtained by candles and lamps.—Place which the discovery of lighting with gas occupies in the philosophical order of knowledge. | ||
| HISTORICAL SKETCH OF THE RISE AND PROGRESS OF THE APPLICATION OF COAL-GAS AS A SUBSTITUTE FOR PROCURING ARTIFICIAL LIGHT. | 55. | |
| The discovery of the inflammable nature and application of coal-gas for the production of artificial light, cannot be claimed by any body now living.—Early notices of the inflammable property of the gas obtained by distilling coal.—Attempts to substitute it for tallow and oil.—Experiments made with coal-gas by Dr. Clayton, Dr. Hales, and the Bishop of Llandaff.—First successful attempt of lighting manufactories with gas.—Creditor and debtor account concerning the expence of this mode of illumination, when compared with the light obtained by tallow candles.—Claims of Mr. Murdoch with regard to the economical application of coal-gas.—Claims of Mr. Winsor.—Experiments of Mr. Northern, Mr. Clegg, Mr. Cook, Mr. Ackermann.—Economical statements of the gas-light illumination when compared with the cost of the same quantity of light obtained by means of candles and lamps. | ||
| THEORY OF THE PRODUCTION OF GAS-LIGHT; AND DESCRIPTION OF A PORTABLE APPARATUS FOR ILLUSTRATING, IN THE SMALL WAY, THE GENERAL NATURE OF THE NEW SYSTEM OF PROCURING LIGHT.[iii] | 77. | |
| Philosophy of the production of coal-gas.—Characters of the various products which the gas-light process affords, their quantities, and modes of obtaining them.—Quantity of gas obtainable from a given weight of coal.—Illuminating power of a given bulk of coal-gas compared with the illuminating power of a given weight of tallow candles.—Practical directions with regard to the production of the gas from coal.—Its chemical constitution and analysis.—Pit-coal is not the only substance which affords carburetted hidrogen gas.—This gas exists ready formed in nature.—Mode of collecting it when found native.—Is given out by all kinds of vegetable matter, submitted to distillation in close vessels.—Other sources of obtaining this gazeous fluid.—Practical directions with regard to the method of obtaining from coal, this gazeous substance, as best suited for illumination.—Chemical constitution of coal-gas.—How ascertained. | ||
| UTILITY OF THE GAS-LIGHT ILLUMINATION WITH REGARD TO PUBLIC AND PRIVATE ECONOMY. | 99. | |
| Objects to which the new system of lighting with gas may be beneficially applied.—Capital advantages of the gas-light illumination.—Places and public edifices lighted with coal-gas in this metropolis.—Situations best suited for the application of gas-lights.—places where it cannot be used to advantage.—Illumination of barracks, arsenals, dock yards, &c. with coal-gas.—Further observations on this subject.—Great heat produced by gas-lights.—Reason why the flame of coal-gas produces more heat than the flame of candles and lamps.—Admeasurement of the comparative degrees of heat produced by gas-lights, oil lamps, tallow and wax candles, &c.—Gas lamps and burners, various kinds of.—Ornamental chandeliers and candelabras, for applying coal-gas as a substitute for oil.—Other products obtainable from coal besides gas.—Coke.—Its nature.—Combustion of it.—Produces a more strong and lasting heat than coal.—Explanation of this fact.—Advantages resulting from the use of coke as fuel.—Disadvantages of its application in certain circumstances.—Relative effect of heat produced by equal quantities of coke and charcoal.—Method of measuring the comparative effect of different kinds of fuel in producing heat.—Capital advantages resulting from the application of coke, as fuel, in the art of burning lime.—Plaster of Paris, bricks, &c.—Quantity of coke obtainable from [iv]a certain quantity of pit-coal.—Kind of coke best suited for metallurgical operations.—Mode of obtaining it in the gas-light process.—Sort of coke best adapted for kitchen and parlour fires.—Manufacture of it.—Coal tar.—How obtained.—Its properties.—Earl of Dundonald’s method of manufacturing tar from coal.—Quantity of coal-tar produced in the gas-light process from a given quantity of coal.—Characters of coal tar obtained from Newcastle coal, differ from that produced from canel coal.—Coal pitch.—Process for obtaining it.—Properties of coal-pitch.—Use of it in the arts.—quantity of coal-pitch obtainable from a given quantity of tar.—Ammoniacal liquor produced during the distillation of coal.—Its chemical constitution.—Quantity obtained from a given quantity of coal.—General observation respecting the scheme of applying coal-gas as a substitute for candles and lamps.—Effects which it must produce upon the arts and upon domestic economy.—Its views.—Primary advantages.—Resources which it presents to industry and public economy.—In what respect it is entitled to public approbation and national encouragement.—Effects of prejudice against the introduction of new and useful discoveries.—Have operated strongly in retarding the gas-light illumination.—Remarkable slowness with which improvements of extended utility make their way into common use, contrasted with the rapid adoption of fashionable changes.—Other causes unfavourable to the adoption of new and useful plans.—Further observations on this subject.—The new system of lighting with coal-gas can never supersede the use of candles and moveable lights.—Gas-light illumination cannot prove injurious to the Greenland fishery—nor can it diminish the coal trade—must prove beneficial to it.—The price of coal even when it is the highest cannot materially affect the beneficial application of gas-lights.—Striking advantages to be derived from the introduction of gas-lights into manufactories.—Principal expense which must always attend the gas-light illumination.—Is the dead capital employed for erecting the machinery.—Floating capital is small.—Advice to private individuals with regard to the erection of a gas-light apparatus calculated for their own use.—Expence which must attend the application of the new system of lighting under different circumstances.—Entire new scheme of illuminating streets, or small towns, with gas-lights; which would save all the main pipes for conveying the gas through the streets as well as the branch pipes which conduct the gas to the lamps.—Management of the gas-light machinery is extremely simple and easy.—The apparatus not liable to be out of order.—Observations on the safety of the gas-light illumination.—Misapprehension of the public concerning it.—Causes that have alarmed the public concerning the application of the new lights.—Gas-lights cannot give rise to those accidents which have so often arisen from the careless snuffing of candles, [v]&c.—Produce no embers or sparks.—Cannot fall, or be disturbed without becoming extinguished.—Are the safest of all lights.—Impossibility of streets or towns lighted with gas to be thrown suddenly into darkness by the fracture of the gas-pipes conveying the gas to the lamps—or by the destruction of one or more of the gas-light machineries employed for preparing the gas.—Illustration showing the absurdity of such mistaken notions.—Curious self-extinguishing lamp, invented by Mr. Clegg.—His machine which measures and registers in the absence of the observer, the quantity of gas delivered by a pipe communicating with a gas-light main.—Leading characters of the new lights.—Objects and views which this art embraces.—It must lessen the consumption of oil.—Occasion a defalcation in the revenue. | ||
| TABULAR VIEW, Exhibiting the quantity of Gas, Coke, Tar, Pitch, Essential Oil, and Ammoniacal Liquor, obtainable from a given quantity of Coal: together with an estimate of the quantity of Coal necessary to produce a quantity of Gas, capable of yielding a Light equal in duration of time and intensity to that produced by Tallow Candles of different kinds. | 164. | |
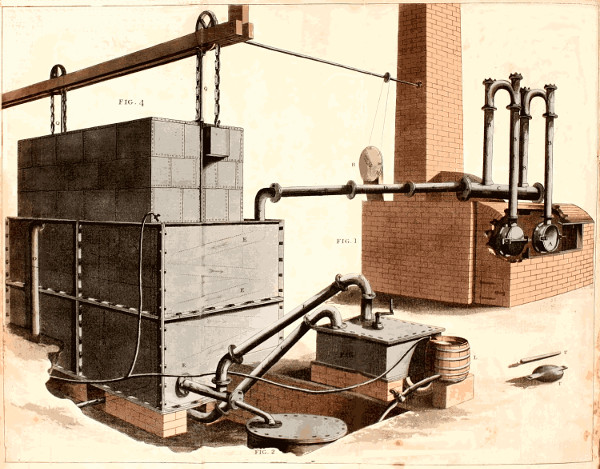
| DESCRIPTION OF THE GAS-LIGHT APPARATUS. | 166. | |
| METHOD of correcting the relative pressure of the Gasometer, so as to cause the gas which it contains to be uniformly of an equal density. | 181. | |
| DIRECTIONS TO WORKMEN ATTENDING THE GAS-LIGHT APPARATUS. | 182. | |
| ESTIMATE of the price of a Gas-Light Apparatus. | 185. | |
| LONDON Price List of the most essential articles employed in the erection of a Gas-light Apparatus. | 186. | |
| Page | 24, | line | 11, | for too, read two. |
| 48, | 22, | for corporated, read incorporated. | ||
| 53, | 7, | for this combustion, read the combustion. | ||
| 64, | 24, | for Cleg, read Clegg. | ||
| ibid | 25, | for communicates, read communicated. | ||
| 65, | erase the * and put it after the word Clegg, line 24, p. 64. | |||
| ibid | 17, | for attemps, read attempts. | ||
| 125, | 23, | for degree, read degrees. | ||
| 132, | 25, | for coal, read coal-tar. | ||
Plate I. facing the title; plate II. facing page 79; plate III. facing page 115; plate IV. facing page 119; plate V. facing page 120; and plates VI. and VII. at the end of the book.
A
PRACTICAL TREATISE
ON
GAS-LIGHT.
It is an undoubted truth, that the successive improvements in the condition of man, from a state of ignorance and barbarism, to that of the highest cultivation and refinement, are usually effected by the aid of machinery and expedients, calculated to procure the necessaries, the comforts, and the elegancies of life; and that the pre-eminence of any people in[2] civilization is, and ought ever to be, estimated by the proportional state of industry, and useful labour existing among them.
In proof of this great and striking truth, no other argument requires to be offered, than an immediate reference to the experience of all ages and places: the various nations of the earth, the provinces of each nation, the towns, and even the villages of the same province, differ from each other in their accommodations; and are in every respect more flourishing, the greater their activity in establishing new channels of useful employ, calculated to procure the necessaries and comforts of life. Hence the nations which have shewn the most ingenuity in this way, are not only the richest, but also the most populous and the best defended: the provinces of those nations, are seen to flourish likewise in proportion to their respective degrees of activity in this respect, And from these exertions it is, as Smith[1] emphatically remarks, that “the accommodation of an European prince does not always so much exceed that of an industrious[3] and frugal peasant, as the accommodation of the latter exceeds that of many an African king, the absolute master of the lives and liberties of ten thousand naked savages.”
[1] Wealth of Nations, chap. 1.
It was a strange notion of Rousseau to maintain that mankind were happier when they resembled wild beasts, than with all the expanded knowledge of civilized life; and that the cultivation of their understanding had tended to degenerate their virtues. There can be no virtue but what is founded on a comprehensive estimate of the effects of human actions, and an animal under the guidance of instinct can form no such estimate.
The variety of production, of wants, and fabrication of a civilized society, has given rise to barter or exchange; mutual supply has increased the sub-division of labour, and improved the means of conveyance. Streams, roads, ships, and carriages have extended their beneficial intercourse; confidence between man and man has advanced the moral principles of society, and afforded a progression, of which the past gradation may indeed be traced, but to the future part of which the[4] imagination can scarcely form a probable outline. And as the moral and physical powers of man expand, new resources and new agencies are made subservient to our commands, which, in an earlier state of society, would have appeared altogether visionary.
Who among the ancients would have listened to the extraordinary scheme of writing books with such rapidity, that one man, by this new art, should perform the work of twenty thousand amanuenses? What philosopher would have given credit to the daring project of navigating the widest ocean?—or imagined the astonishing effect of gun-powder—or the extended application of the steam engine? What mortal would have dared to dive to the bottom of the sea—or to soar aloft into the air—or bid defiance to the thunder of the clouds? Discoveries which have changed, as it were, the course of human affairs, and the effects of which have already carried the intellectual operations of the human mind, to a height they could by no other means have attained. The men of those early ages, in the confidence of their own wisdom, might have derided these discoveries as impossible, or rejected[5] them as visionary; but to those, who enjoy the full effects of such, and numerous other successful inventions, it becomes a duty to reason upon different principles, and to exert all means in their power to give effect to the progress of useful knowledge.
The artificial production and supply of light during the absence of the sun, unquestionably holds a distinguished rank among the most important arts of civilized life.
If we could for a moment suppose the privation of artificial light, it would follow as an immediate consequence that the greatest part of the globe on which we dwell, would cease to be the habitation of man. Whether he could ensnare or overtake those animals upon whose unprepared remains he would then be compelled to feed—whether he might store the fruits of the earth for his winter supply—what might be the physical and moral consequences of a state of such desolation, may perhaps be conjectured; but no estimate can show its dreadful magnitude. How much do our comforts, and how greatly does the extent of our powers, in the common affairs of life, depend upon the production and supply[6] of artificial light. The flame of a single candle animates a family, every one follows his occupation, and no dread is felt of the darkness of night. It might be a curious speculation to enquire how far, and in what respects, the morals of men would become degraded by the want of this contrivance. But it is sufficient on the present occasion, that, previous to entering upon a dissertation respecting a new art of illumination, a train of ideas has slightly been hinted at, which cannot fail to show its magnitude and importance. The methods of procuring and distributing light, during the absence of the sun, have not hitherto attained the extent of their possible perfection: there is yet a wide field for improvement in the construction of the instruments of illumination, and the subject is highly deserving the attention of every individual.
The scheme of lighting houses, streets, and manufactories, by means of the inflammable gas, obtainable by distillation from common pit-coal, professes to increase the wealth of the nation, by adding to the number of its internal resources, and on this ground it is entitled, at least, to a candid examination.
The apparent slight that has been thrown upon this new breach of civil economy by some individuals, who appear to be incapable of judging of its nature, has contributed to deter sensible and well disposed persons from wishing it success. It is the more necessary to state this fact, because, when a mistaken notion once becomes diffused, concerning the nature of a new project, persons of the best intention are liable to become affected with wrong impressions on their mind. I am neither a share holder, nor a governor, nor am I directly or indirectly concerned in any gas-light association.
The object of the succeeding pages, simply is to rescue the art of illumination with coal-gas from misconception and misrepresentation, and by a fair, and not overcharged statement of its merits and its disadvantages, to appeal from prejudice and ignorance, to the good sense of the community.
The flame of burning bodies consists of such inflammable matter in the act of combustion as is capable of existing in a gazeous state. When all circumstances are favorable to the complete combustion of the products, the flame is perfect; if this is not the case, part of the combustible body, capable of being converted into the gazeous state, passes through the luminous flame unburnt, and exhibits the appearance of smoke. Soot therefore always indicates an imperfect combustion. Hence[9] flame is produced from those inflammable substances only, which are either totally volatile when heat is applied to them, so as not to alter their chemical habitudes—or which contain a quantity of combustible matter that is readily volatilized into vapour by heat, or the elements necessary for producing such vapour or gazeous products, when the chemical constitution of the body is altered by an increase of temperature. And hence the flame of bodies is nothing else than the inflammable product, either in a vaporous or in a permanently elastic gazeous state. Thus originates the flame of wood and coal, when they are burned in their crude state. They contain the elements of a quantity of inflammable matter, which is capable of assuming the gazeous state by the application of heat, and subsequent new chemical arrangements of their constituent parts.
As the artificial light of lamps and candles is afforded by the flame they exhibit, it seems a matter of considerable importance to society, to ascertain how the most luminous flame may be produced with the least consumption of combustible matter. There does not appear[10] to be any danger of error in concluding, that the light emitted will be greatest when the matter is completely consumed in the shortest time. It is therefore necessary, that the stream of volatilized combustible gazeous matter should pass into the atmosphere with a certain determinate velocity. If the quantity of this stream should not be duly proportioned; that is to say, if it be too large, its internal parts will not be completely burned for want of contact with the air. If its temperature be below that of ignition, it will not, in many cases, burn when it comes into the open air. And there is a certain velocity at which the quantity of atmospherical air which comes in contact with the vapour will be neither too great nor too small; for too much air will diminish the temperature of the stream of combustible matter so much as very considerably to impede the desired effect, and too little will render the combustion languid.
We have an example of a flame too large in the mouths of the chimneys of furnaces, where the luminous part is merely superficial, or of the thickness of about an inch or two, according to circumstances, and the internal part,[11] though hot, will not set fire to paper passed into it through an iron tube; the same defect of air preventing the combustion of the paper, as prevented the interior fluid itself from burning. And in the lamp of Argand we see the advantage of an internal current of air, which renders the combustion perfect by the application of air on both sides of a thin flame. So likewise a small flame is always whiter and more luminous than a larger; and a short snuff of a candle giving out less combustible matter in proportion to the circumambient air; the quantity of light becomes increased to eight or ten times what a long snuff would have afforded.
The light of bodies burning with flame, exists previously either combined with the combustible body, or with the substance which supports the combustion. We know that light exists in some bodies as a constituent part, since it is disengaged from them when they enter into new combinations, but we are unable to obtain in a separate state the basis with which it was combined.
That in many cases the light evolved by artificial means is derived from the combustible[12] body, is obvious, if we recollect that the colour of the light emitted during the process of combustion varies, and that this variation usually depends not upon the medium which supports the process of combustion, but upon the combustible body itself. Hence the colour of the flame of certain combustibles, even of the purest kind may be tinged by the admixture of various substances.
The flame of a common candle is far from being of an uniform colour. The lowest part is always blue; and when the flame is sufficiently elongated, so as to be just ready to smoke, the tip is red or brown.
As for the colours of flames that arise from coals, wood, and other usual combustibles, their variety, which hardly amounts to a few shades of red or purple, intermixed with the bright yellow light, seems principally to arise from the greater or less admixture of aqueous vapour, dense smoke, or, in short, of other incombustible products which pass through the luminous flame unburnt.
Spirit of wine burns with a blueish flame. The flame of sulphur has nearly the same tinge. The flame of zinc is of a bright greenish[13] white. The flame of most of the preparations of copper, or of the substances with which they are mixed, is vivid green. Spirit of wine, mixed with common salt, when set on fire, burns with a very unpleasant effect, as may be experienced by looking at the spectators who are illuminated by such light. If a spoonful of spirit of wine and a little boracic acid, or nitrate of copper be stirred together in a cup, and then be set on fire, the flame will be beautifully green. If spirit of wine be mixed with nitrate of strontia, it will, afterwards, on being inflamed, burn with a carmine red colour. Muriate of lime tinges the flame of burning spirit of wine of an orange colour.[2]
[2] See Chemical Amusement, comprising minute instructions for performing a series of striking and interesting chemical experiments, p. 8, &c.
Before we consider the general nature of Gas-Light, it will be necessary to give a short sketch of the theory and action of the instruments of illumination employed for supplying light, together with some other facts connected with the artificial production and distribution of light; such a proceeding will enable us to understand the general nature of[14] the new system of illumination which it is the object of this Essay to explain.
To procure light for the ordinary purposes of life, we are acquainted with no other ready means than the process of combustion.
The rude method of illumination consists, as is sufficiently known, in successively burning certain masses of fuel in the solid state: common fires answer this purpose in the apartments of houses, and in some light-houses. Small fires of resinous wood, and the bituminous fossil, called canel-coal, are in some countries applied to the same end, but the most general and useful contrivance is that in which fat, or oil, of an animal or vegetable kind is burned by means of a wick, and these contrivances comprehend candles and lamps.
In the lamp the combustible substance must be one of those which retain their fluidity at the ordinary temperature of the atmosphere. The candle is formed of a material which is not fusible but at a temperature considerably elevated.
All these substances must be rendered volatile before they can produce a flame, but for this purpose it is sufficient to volatilize a small[15] quantity of any of them, successively; for this small quantity will suffice to give a useful light, and hence we must admire the simple, yet wonderful contrivance of a common candle or lamp. These bodies contain a considerable quantity of the combustible substance, sufficient to last several hours; they have likewise, in a particular place, a slender piece of spongy vegetable substance, called the wick, which in fact is the fire-place, or laboratory where the whole operation is conducted.
There are three articles which demand our attention in the lamp—the oil, the wick, and the supply of air. It is required that the oil should be readily inflammable; the office of the wick appears to be chiefly, if not solely, to convey the oil by capillary attraction to the place of combustion; as the oil is decomposed into carburetted hydrogen gas and other products, other oil succeeds, and in this way a continual current and maintenance of flame is effected.
When a candle is for the first time lighted, a degree of heat is given to the wick, sufficient first to melt, and next to decompose the tallow surrounding its lower surface; and just[16] in this part the newly generated gas and vapour is, by admixture with the air, converted into a blue flame; which, almost instantaneously encompassing the whole body of the vapour, communicates so much heat to it, as to make it emit a yellowish white light. The tallow now liquefied, as fast as it boils away at the top of the wick, is, by the capillary attraction of the same wick, drawn up to supply the place of what is consumed by the cotton. The congeries of capillary tubes, which form the wick, is black, because it is converted into coal; a circumstance common to it with all other vegetable and animal substances, when part of the carbon and hydrogen which enter into their composition having been acted on by combustion, the remainder and other fixed parts are by any means whatever covered and defended from the action of the air. In this case, the burning substance owes its protection to the surrounding flame. For when the wick, by the continual wasting of the tallow, becomes too long to support itself in a perpendicular situation, the top of it projects out of the cone formed by the flame, and thus being exposed to the action of the air, is ignited,[17] loses its blackness, and is converted into ashes; but that part of the combustible which is successively rendered volatile by the heat of the flame is not all burnt, but part of it escapes in the form of smoke through the middle of the flame, because that part cannot come in contact with the oxygen of the surrounding atmosphere; hence it follows, that with a large wick and a large flame, this waste of combustible matter is proportionately much greater than with a small wick and a small flame. In fact, when the wick is not greater than a single thread of cotton, the flame, though very small, is, however, peculiarly bright, and free from smoke; whereas in lamps, with very large wicks, such as are often suspended before butchers’ shops, or with those of the lamp-lighters, the smoke is very offensive, and in great measure eclipses the light of the flame.
A candle differs from a lamp in one very essential circumstance; viz. that the oil or tallow is liquefied, only as it comes into the vicinity of the combustion; and this fluid is retained in the hollow of the part, which is still concrete, and forms a kind of cup. The[18] wick, therefore, should not, on this account, be too thin, because if this were the case, it would not carry off the material as fast as it becomes fused; and the consequence would be, that it would gutter or run down the sides of the candle: and as this inconvenience arises from the fusibility of the tallow it is plain that a more fusible candle will require a larger wick; or that the wick of a wax candle may be made thinner than that of one of tallow. The flame of a tallow candle will of course be yellow, smoky, and obscure, except for a short time after snuffing. When a candle with a thick wick is first lighted, and the wick snuffed short, the flame is perfect and luminous, unless its diameter be very great; in which last case, there is an opake part in the middle, where the combustion is impeded for want of air. As the wick becomes longer, the interval between its upper extremity and the apex of the flame is diminished; and consequently the tallow which issues from that extremity, having a less space of ignition to pass through, is less completely burned, and passes off partly in smoke. This evil increases, until at length the upper extremity of the wick projects beyond[19] the flame and forms a support for an accumulation of soot which is afforded by the imperfect combustion, and which retains its figure, until, by the descent of the flame, the external air can have access to the upper extremity; but in this case, the requisite combustion which might snuff it, is not effected; for the portion of tallow emitted by the long wick is not only too large to be perfectly burned, but also carries off much of the heat of the flame, while it assumes the elastic state. By this diminished combustion, and increased afflux of half decomposed oil, a portion of coal or soot is deposited on the upper part of the wick, which gradually accumulates, and at length assumes the appearance of a fungus. The candle then does not give more than one-tenth of the light which the due combustion of its materials would produce; and, on this account, tallow candles require continual snuffing. But if we direct our attention to a wax candle, we find that as its wick lengthens, the light indeed becomes less. The wick, however, being thin and flexible, does not long occupy its place in the centre of the flame; neither does it, even in that situation, enlarge[20] the diameter of the flame, so as to prevent the access of air to its internal part. When its length is too great for the vertical position, it bends on one side; and its extremity, coming in contact with air, is burned to ashes; excepting such a portion as is defended by the continual afflux of melted wax, which is volatilized, and completely burned, by the surrounding flame. Hence it appears, that the difficult fusibility of wax renders it practicable to burn a large quantity of fluid by means of a small wick, and that this small wick, by turning on one side in consequence of its flexibility, performs the operation of snuffing itself, in a much more accurate manner than can ever be performed mechanically. From the above statement it appears, that the important object to society of rendering tallow candles equal to those of wax, does not at all depend on the combustibility of the respective materials, but upon a mechanical advantage in the cup, which is afforded by the inferior degree of fusibility in the wax: and that, in order to obtain this valuable object, one of the following effects must be produced: either the tallow must be burned in a lamp, to avoid the[21] gradual progression of the flame along the wick; or some means must be devised to enable the candle to snuff itself, as the wax-candle does; or the tallow itself must be rendered less fusible by some chemical process. The object is, in a commercial point of view, entitled to assiduous and extensive investigation. Chemists in general suppose the hardness or less fusibility of wax to arise from oxygen. Mr. Nicholson[3] is led by various considerations to imagine, that the spontaneous snuffing of candles made of tallow or other fusible materials, will scarcely be effected but by the discovery of some material for the wick, which shall be voluminous enough to absorb the tallow, and at the same time sufficiently flexible to bend on one side.
[3] Philosophical Journal, 4to Series, Vol. I. p. 70.
Though the eye is not fitted to judge of the proportional force of different lights, it can distinguish, in many cases with great precision, when two similar surfaces, presented together, are equally illuminated. But as the lucid particles are darted in right lines, they must spread uniformly, and hence their density will diminish in the duplicate ratio of their distance. From the respective situations, therefore, of the centres of divergency, when the contrasted surfaces become equally bright, we may easily compute their relative degrees of intensity.
For this purpose it is assumed as a principle, that the same quantity of light, diverging in[23] all directions from a luminous body, remains undiminished in all distances from the centre of divergency. Thus we must suppose, that the quantity of light falling on every body, is the same as would have fallen on the places occupied by the shadow; and if there were any doubt of the truth of the supposition, it might be confirmed by some simple experiment. Therefore, it follows, that, since the shadow of a square inch of any surface occupies at twice the distance of the surface from the luminous point the space of four square inches, the intensity of the light diminishes as the square of the distance increases. If, consequently, we remove two sources of light to such distances from an object that they may illuminate it in equal degrees, we may conclude that their original intensities are inversely as the squares of the distances.
Hence, if two lights of unequal illuminating powers shine upon the same surface at equal obliquities, and an opake body be interposed between them and the illuminated surface, the two shadows produced, must differ in blackness or intensity in the same degree. For the shadow formed by intercepting the[24] greater light, will be illuminated by the smaller light only, and reversely the other shadow will be illuminated by the greater light: that is to say, the stronger light will be attended with the deeper shadow. Now it is easy, by removing the stronger light to a greater distance, to render the shadow which it produces at the common surface equal to that afforded by the less. Experiments of this kind may be conveniently made by fastening a sheet of white paper against the wall of a room; the two lights, of whatever nature they are, intended to be compared, must then be placed so that the ray of light from each shall fall with nearly the same angle of incidence upon the middle of the paper. In this situation, if a book or other object be held to intercept part of the light which would have fallen on the paper, the two shadows may be made to appear as in this figure;

where A represents the surface illuminated by one of the lights only; B, the surface illuminated by the other light; C, the perfect shadow from which both lights are excluded. It will easily be understood that the lights about D and E, near the angle F, will fall with equal incidences when the double shadow is made to occupy the middle of the paper; and consequently, if one or both of the lights be removed directly towards or from the paper, as the appearances may require, until the two shadows at E and D have the same intensity, the quantities of light emitted by each will be as the squares of the distances from the paper. By some experiments made in this way, the degree of illumination of different lights may readily be ascertained to the tenth part of the whole. And, by experiments of this kind, many useful particulars may be shewn. For, since the cost and duration of candles, and the consumption of oil in lamps, are easily ascertainable, it may be shewn whether more or less light is obtained at the same expence during a given time, by burning a number of small candles instead of one or more of greater thickness. It will therefore[26] be easy to compare the power of different kinds of lamps or candles, or gas lights, so as to determine the relative cost of each particular kind of the combustible substance employed for furnishing light:—for example, if a candle and a gas-burner supplying coal-gas, adjusted by a stop-cock, produce the same darkness of shadow, at the same distance from the wall, the strength or intensity of light is the same. An uniform degree of intensity of the gas-light may readily be produced, by opening or shutting the stop-cock, if more or less be required, and the candle is carefully snuffed to produce the most regular and greatest quantity of light. The size of the flame in experiments of this kind of course becomes unnecessary, and will vary very much with the quality of the coal gas. The bulk of the gas consumed, and the quantity of tallow used, by weighing the candle before and after the experiment, furnish the data for ascertaining the relative costs of tallow and gas-light, when compared with each other.
From experiments made by Count Rumford, concerning the quantity of materials requisite for producing a light of a certain intensity for[27] a given time: it was found that we must burn of wax 100, of tallow 101, of oil, in an Argand’s lamp, 129, of an ill-snuffed tallow candle 229 parts, by weight. And with regard to the quantity of carburetted hydrogen, or coal-gas, I have found that from 18 to 20 cubic feet (according to the purity of the gas) are required to give a light equal in duration and in illuminating powers to 1lb. of tallow candles, six to the pound, provided they were set up and burnt out one after another.[4]
[4] 112lbs. of Newcastle coal, called Tanfield Moor, produce, upon an average, from 250 to 300 cubic feet of gas, fit for illumination.
It is sufficiently known that the light of a candle, which is so exceedingly brilliant when first snuffed, is very speedily diminished to[28] one-half and is usually not more than one-fifth or one-sixth before the uneasiness of the eye induces us to snuff it.[5] Whence it follows, that if candles could be made so as not to require snuffing, the average quantity of light afforded by the same quantity of combustible matter would be more than doubled.
[5] Ezekiel Walker.—Nicholson’s Journal, Vol. IV. 8vo. Series.
When a lighted candle is so placed as neither to require snuffing or produce smoke, it is reasonable to conclude that the whole of the combustible matter which is consumed is converted to the purpose of generating light; and that the intensities of light afforded in a given time, by candles of different dimensions, are in proportion to the quantity of matter consumed. That is to say; when candles are made of the same materials, if one candle produce twice as much light as another, the former will in the same time lose twice as much weight as the latter.
To prove the truth of this position, Mr. Walker made the experiments contained in the following
TABLE.
| No. of the Experi- ment. |
No. of the Candles. |
Time of burning. |
Weight of the Candles consumed in a given time. |
Strength of Light. |
Distance of the Candles from the Wall. |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| h. | oz. | dr. | Feet. | ||||||||||
| 1 | - | 1 | 3 | 0 | 0 | 15 | 1 | 7 | |||||
| 3 | 3 | 0 | 1 | 1 | 1⁄2 | 1 | + | 7 | |||||
| Mould | 3 | 0 | 0 | 15 | 1 | 7 | |||||||
| 2 | - | 1 | 2 | 55 | 0 | 15 | 1 | 8 | |||||
| 3 | 2 | 55 | 1 | 0 | 1 | + | 8 | ||||||
| Mould | 2 | 55 | 0 | 15 | 1 | 8 | |||||||
| 3 | - | 1 | 3 | 0 | 0 | 15 | 3⁄4 | 1 | 8 | ||||
| 3 | 3 | 0 | 1 | 2 | 1 | 1⁄8 | 8 | 3⁄4 | |||||
| Mould | 3 | 0 | 0 | 0 | 1 | 9 | |||||||
| 4 | - | 5 | 3 | 0 | 1 | 5 | 1 | .18 | 8 | 3⁄4 | |||
| Mould | 3 | 0 | 1 | 1 | 1⁄8 | 1 | . | 8 | |||||
These experiments, Mr. Walker informs us, were made in the following manner:—
Three candles, the dimensions of which are given in the table, against 1, 3, and mould. These were first weighed, and then lighted at the same instant. At the end of the time inserted in the third column of the above table, they were extinguished and weighed again,[30] and the loss of weight of each candle is contained in the fourth column.
The three first experiments were made under such favourable circumstance, that there was little doubt of their results being more accurate than what practical utility requires, but the fourth experiment cannot be depended on so much, in consequence of the variable light of No. 5. This candle was moved so often to keep the two shadows equal, that it was found necessary to set down its mean distance from the wall by estimation; but as this was done before the candles were weighed, the experimenter’s mind could not be under the influence of partiality for a system.
The method which Mr. Walker employed in comparing one light with another in each experiment, was that which has been described page 24.
1. The experiments were made at different times, and the light of the mould candle was made the standard, with which the lights of the others were compared; but it must not be understood, that this candle gave the same strength of light in every experiment.
2. The sign + in the 5th column, signifies[31] that the candle against which it is placed, gave a stronger light than the others.
From the experiments contained in the table, it appears to be an established law, where combustion is complete, that the quantities of light produced by tallow candles, are in the complicate ratio of their times of burning and weights of matter consumed.
For if their quantities of matter be equal, and times of burning the same, they will give equal quantities of light, by the experiments.
And if the times of burning be equal, the quantities of light will be directly as their weights of matter expended.
Therefore the light is universally in the compound ratio of the time of burning and weight of matter consumed.
If the law which Mr. Walker has endeavoured to prove, both by reason and experiment, be admitted, we have a standard with which we may compare the strength of any other light.
Let a small mould candle, when lighted, be so placed as neither to produce smoke nor require snuffing, and it will lose an ounce of its weight in three hours. Let this quantity[32] of light produced under these circumstances, be represented by 1.00.
Then should this candle at any other time, lose more or less of its weight in three hours than an ounce, the quantity of light will be still known, because the quantity of light in a given time is directly as the weight of the candle consumed.[6]
[6] To investigate rules for this purpose, 1. Let M represent the mould candle, a its distance from the wall, on which the shadows were compared, x its quantity of matter consumed in a given time, (t) and Q the quantity of light emitted by M in the same time: 2. Let m represent any other candle, b its distance from the same wall, and y its quantity of matter consumed, in the time t.
Then as the intensities of light are directly as the squares of the distances of the two candles from the wall, we have as a2 : Q ∷:: b2 : b2 + Qa2 = the quantity of light, emitted by m in the time.
Then let us suppose that the quantities of light are directly as the quantities of matter consumed in the time t, and we have, As x : Q ∷:: y : y + Qx = the quantity of light emitted by m in that time, by hypothesis.
Now, when b2 + Qa2 (Theo. 1.) is = Y + QX (Theo. 2.) the quantities of light of M and m are directly as their quantities of matter consumed in any given time.
Mr. Ezekiel Walker has shewn that, if a trifling alteration be made in the method of using common tallow candles, they will become excellent substitutes for those of wax.
A common candle, weighing one-tenth of a pound, containing fourteen single threads of fine cotton, placed so as to form an angle of 30 degrees[7] with the perpendicular, and lighted, requires no snuffing; and what is much more valuable for some purposes, it gives a light that is nearly uniform in strength without the least smoke. These effects are thus produced:
[7] Candlesticks may be made to hold the candle at this angle, or they may be so contrived as to hold the candle at any angle at pleasure.
When a candle burns in an inclined position, most part of the flame rises perpendicularly from the upper side of the wick, and when viewed in a certain direction, it appears in the form of an obtuse angled triangle. And as the end of the wick projects beyond the flame at the obtuse angle, it meets with the air, and is completely burnt to ashes: hence it is rendered incapable of acting as a conductor to carry off part of the combustible matter in the form of smoke. By this spontaneous mode of snuffing, that part of the wick which is acted upon by the flame continues of the same length, and the flame itself very nearly of the same strength and magnitude[8].
[8] The wick’s not being uniformly twisted throughout, may occasion a little variation in the dimensions of the flame.
The advantages which may be derived from candles that require no snuffing and afford no smoke, may be readily understood; but these candles have another property which ought not to be passed over in silence. A candle snuffed by an instrument gives a very fluctuating light, which, in viewing near objects is highly injurious to the eye; and this is an[35] inconvenience which no shade can remove. But when a candle is snuffed spontaneously, it gives a light so perfectly steady and so uniformly bright, that the adjustments of the eye remain at rest, and distinct vision is performed without pain, and without uneasiness.
Candles, on which Mr. Walker has made experiments, are described in the following
TABLE.
| No. | No. of candles to the pound avoir- dupoise weight. |
Length in inches. |
No. of single threads of fine cotton in the wick. |
|
|---|---|---|---|---|
| 1 | 14 | 8. | 5 | 10 |
| 2 | 13 | 9. | 12 | |
| 3 | 10 | 9. | 74 | 14 |
| 4 | 8 | 10. | 20 | |
| 5 | 6 | 10. | 25 | 24 |
| Mould | 6 | 13. | ||
Number 1, 2, and 3. These candles, when lighted and placed to form an angle of 30° with the perpendicular, require no snuffing: they give lights which are nearly equal, and combustion proceeds so regularly, that no part of the melted tallow escapes unconsumed, except from accidental causes.
No. 4, placed at the angle mentioned above, and lighted, requires no snuffing: it gives a[36] light very little stronger than No. 1, but its colour is not quite so white, nor its flame so steady.
No. 5. This candle, placed at an angle of 30°, and lighted, requires no snuffing; its flame is rather fluctuating, and not so white as No. 4, nor is its strength of light much greater than No. 1. The melted tallow sometimes overflows when the air in the room is put in motion; yet the light of this candle is much improved by being placed in an inclined position.
The mould candle, treated in the same manner, affords a very pure steady flame, without smoke and without snuffing, and its strength of light is about equal to that of No. 1.
The experiments have not been sufficiently numerous to determine with precision which of these candles affords the most light at a given expence, but the few experiments which have been made seem to indicate, that the quantity of light is nearly as the quantity of combustible matter consumed, and thus a candle which is used in the manner pointed out gives more light than a candle of the same dimension set perpendicularly and snuffed,[37] because one part of a candle that is snuffed, is thrown away, and another part flies off in the form of smoke. And this is not the only inconvenience that attends the using candles in this manner, and which the other method is free from, for the light which it gives is of a bad quality, on account of its being variable and undulating.
From the time that a candle is snuffed till it wants snuffing again, its strength of light scarcely continues the same for a single minute. And that variation which frequently takes place in the height of the flame, is a matter of still more serious consequence.
The flame of a long candle placed vertically when it is snuffed burns steadily, is about two inches high, but it very frequently rises to the height of four inches or upwards; drops down again in a moment, till it is less than three inches, and then rises again. In this manner the flame continues in motion for some time before it returns to its original dimensions. But it does not continue long in a quiescent state before it begins a new series of undulations. In this manner the candle burns till the top of the wick is seen near the apex of[38] the flame, carrying off clouds of smoke. In this state of things the eye becomes uneasy for want of light, and the snuffers are applied to remove the inconvenience.
Mr. Walker further observes, that it is these sudden changes, and not the nature of candle-light itself, that do so much injury to the eye of the student and artist; and that that injury may be easily prevented, by laying aside the snuffers, and in the place of one large candle, let two small ones be used in the manner stated.
The following observations on this subject are copied from the Monthly Magazine, 1805, p. 206.
“It is scarcely necessary to observe, that the combustion of candles proceeds the quicker in proportion as the inclination is greater. From the experiments which I have made, I should consider an angle of forty degrees with the perpendicular as the maximum of inclination, beyond which several considerable inconveniencies would occur; and I should take 25 degrees as the minimum of inclination, less than which does not sufficiently expose the point of the wick to the action of the air.
“By those who are much in the habit of reading or writing by candle-light, it will also be esteemed no inconsiderable addition to the advantages already mentioned, that the trouble of seeking and applying the snuffers is superseded. A candle of common size in a vertical position, requires the application of the snuffers forty-five times during its complete consumption.
“But I found an obstacle to the adoption of Mr. Walker’s plan, which, from the inclined position of the candle, it did not immediately occur to me by what means to counteract. Any agitation of the air of the room, occasioned either by the opening or shutting of a door, or by the quick passage of a person near the candle, caused the melted tallow to run over, or, in more familiar language, caused the candle to gutter; which, with the candle in this position, became an insuperable bar to the use of it.
“For the prevention of this inconvenience, I have had a wire skeleton-shade adapted to a rod bearing the same inclination as the candle, and which at bottom joins the candlestick in an horizontal line of about two inches, terminating[40] in a nozzle fitting that of the candlestick.—The distance of this rod from the candlestick, or, which is the same thing, the length of the foot or horizontal line, is of course to be determined by the distance between the two circles which form the upper and lower apertures of the shade.—It may serve, perhaps, more familiarly to describe this part of the apparatus, to state, that it bears a perfect resemblance to the two first strokes of the written figure 4; and the third stroke, if carried up as high as the first, and made sloping instead of upright, will very well represent the situation of the candle.
“When a strong light, for the purposes of reading or writing, be required, a white silk or paper may be used, as is common, over the skeleton; but when it be required that the light should be dispersed over the room, a glass of a similar shape may be adopted, for the purpose of preventing the flame from being influenced by any agitation of the air of the room. If the upper circle of the shade be four inches in diameter, the apex of the flame will be within it during more than half the time of the complete consumption of the candle; the shade[41] will not, therefore, require adjusting for the purpose of preventing injury to the silk, or whatever else may be used over the skeleton, more than once during that time.
“Being myself much averse to the interruptions which a candle used in a vertical position occasions, and which, though short, may, under some circumstances, be highly vexatious, I wish to extend to others a benefit which I prize rather highly.”
Lord Stanhope[9] has published a simple method of manufacturing candles, which, according to his Lordship’s statement, is superior to the method usually employed. The principles upon which the process depends are the following:—First, the wick of the candle is to have only three-fourths of the usual number of cotton threads, if the candle be of wax or spermaceti; and only two-thirds of the usual number, if the candle be of tallow. Secondly, it is required that the wick in all cases be perfectly free from moisture, a circumstance seldom attended to in the manufacturing of candles; and thirdly, to deprive the wick of wax[42] candles, of all the air which is entangled in its fibres, and this may conveniently be done, by boiling it in melted wax, till no more air bubbles, or froth appear on the surface of the fluid.
[9] Repository of Arts, Vol. I, p. 86.
If these circumstances be attended to, three candles of any size thus prepared, last as long as four of the same size manufactured in the common way. The light which they afford is superior and more steady than the light of common candles; and lastly, candles made in this manner, whether of wax, spermaceti, or tallow, do not require to be snuffed as often. Besides all this, they flame much less, and are consequently better for writing, reading, working and drawing, than candles made by the common method.
The following observations will enable any person who is willing to try the candles manufactured according to Lord Stanhope’s plan, to ascertain the real value of the improvements suggested by his Lordship. It shews also the result of some experiments, made to ascertain the expence of burning oil in lamps with wicks of various sizes.
A taper lamp, with eight threads of cotton, will consume in one hour 225⁄1000 oz. of spermaceti oil: at six shillings per gallon, the expence of burning twelve hours is 13.71 farthings.
At seven shillings, it is 15.995 farthings.
At eight shillings, it is 18.280 farthings.
N. B. This gives as good a light as tallow candles of eight and ten in the pound. This lamp seldom wants snuffing, and casts a steady and strong light.
A taper, chamber, or watch lamp, with four ordinary threads of cotton in the wick, consumes 1.664 oz. of spermaceti oil in one hour: the oil at seven shillings per gallon, the expence of burning twelve hours, 7.02 farthings.
At eight shillings, it is 8.022 farthings.
At nine shillings, it is 9.024 farthings.
TABLE,
Exhibiting a series of experiments, made with a view to determine the real and comparative expence of burning candles of different sorts and sizes.
| Number of candles in one pound. |
Weight of one candle. |
Time one candle lasted. |
The time that one pound will last. |
The expence in twelve hours when candles are at 12s. per dozen, which also shews the proportion of expence at any price, per dozen. |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oz. | Dr. | Hr. | Min. | Hr. | Min. | Farthings and hundredth parts. |
|||||
| A small wick. A large wick. |
18 | 3⁄4 | 0 | 14 | 3 | 15 | 59 | 26 | 9 | .70 | |
| 19 | 0 | 13 | 1⁄2 | 2 | 40 | 50 | 34 | 11 | .40 | ||
| 16 | 1⁄2 | 0 | 15 | 1⁄2 | 2 | 40 | 44 | 2 | 13 | .08 | |
| 12 | 1 | 5 | 1⁄4 | 3 | 27 | 41 | 24 | 13 | .92 | ||
| 10 | 3⁄4 | 1 | 8 | 3 | 36 | 38 | 24 | 15 | .00 | ||
| 7 | 3⁄4 | 2 | 1 | 4 | 9 | 32 | 12 | 17 | .88 | ||
| 8 | 2 | 0 | 4 | 15 | 34 | 0 | 16 | .94 | |||
| 5 | 3⁄4 | 2 | 13 | 5 | 19 | 30 | 15 | 19 | .06 | ||
| Mould candles. |
Moulds at 14d. per dozen. |
||||||||||
| Each. | |||||||||||
| With wax’d wick. |
3 | 7⁄8 | 2 | 12 | 7 | 20 | 42 | 39 | 15 | .74 | |
| 4 | 4 | 0 | 9 | 3 | 36 | 20 | 18 | .56 | |||
| 3 | 5 | 2 | 3⁄4 | 17 | 30 | 52 | 30 | 16 | .825 | ||
The time each candle lasted, was taken from an average of several trials on each size.
It has been suggested by Dr. Franklin, that the flame of two candles joined, gives a much stronger light than both of them separately. The same, has been observed by Mr. Warren, to be the case with flames of gas-lights, which,[45] when combined, give a much stronger light than they would afford, when in a separate state.
Indeed, in all cases, where flames for producing light are placed near to each other, it is always beneficial to preserve the heat of the flame as much as possible. One of the most simple methods of doing this, is no doubt, the placing of the several flames together, and as near as possible to each other without touching, in order that they may mutually cover and defend each other against the powerful cooling influence of the surrounding cold bodies. This principle is now employed in the Liverpool lamp, which acts by several flat or ribband wicks placed in the form of a cylinder. The power of illumination of this lamp is superior in effect and more economical than any other lamp in use—and as flame is perfectly transparent to the light of another flame which passes through it, there is no danger of loss of light on account of the flames covering each other.
A new art of procuring artificial light, which consists in burning the gazeous fluid obtained by distillation from common pit-coal, has of late engaged the attention of the public, under the name of gas-light.
The encouragement that has been given for some years past by the legislature to this system of lighting, has induced certain individuals to apply the coal-gas light for the illumination of streets, houses, roads, and public edifices. And it is sufficiently known that a company has been incorporated by charter under the name of the “Gas Light and Coke Company,” to apply this new art of procuring light, by[48] way of experiment, on a large scale, in lighting the streets of the metropolis.[10]
[10] An Act for granting certain powers and authorities to a company to be incorporated by charter, called the “Gas Light and Coke Company,” for making inflammable air for the lighting of the streets of the metropolis, &c.—Session 1810, 50th Geo. III.
The power and authorities granted to this corporate body are very restricted and moderate. The individuals composing it have no exclusive privilege; their charter does not prevent other persons from entering into competition with them. Their operations are confined to the metropolis where they are bound to furnish not only a stronger and better light to such streets and parishes as chuse to be lighted with gas, but also at a cheaper price than shall be paid for lighting the said streets with oil in the usual manner. The corporation is not permitted to traffic in machinery for manufacturing or conveying the gas into private houses, their capital or joint stock is limited to 200,000l. and His Majesty has the power of declaring the gas-light charter void, if the company fail to fulfil the terms of it.
Pit-coal exists in this island in strata, which, as far as concerns many hundred generations after us, may be pronounced inexhaustible; and is so admirably adapted, both for domestic purposes and the uses of the arts, that it is justly regarded as a most essential constituent of our national wealth. Like all other bituminous substances, it is composed of a fixed carbonaceous base or bitumen, united to more or less earthy and saline matter constituting the ashes left behind when this substance is burnt. The proportions of these parts differ considerably, in different kinds of coal; and according to the prevalency of one or other of them, so the coal is more or less combustible, and possesses the characters of[50] perfect pit-coal; and by various shades, passes from the most inflammable canel-coal, into blind, Kilkenny, or stone-coal; and, lastly, into a variety of earthy or stony substances; which, although they are inflammable, do not merit the appellation of coal.
Every body knows that when pit-coals are burning in our grates, a flame more or less luminous issues from them, and that they frequently emit beautiful streams of flame remarkably bright. But besides the flame, which is a peculiar gas in the state of combustion, heat expels from coal an aqueous vapour, loaded with several kinds of ammoniacal salts, a thick viscid fluid resembling tar, and some gases that are not of a combustible nature. The consequence of which is, that the flame of a coal-fire is continually wavering and changing, both in shape, as well as brilliance and in colour, so that what one moment gave a beautiful bright light, in the next, perhaps, is obscured by a stream of thick smoke.
But if coals, instead of being suffered to burn in this way, are submitted to distillation in close vessels, all its immediate constituent parts may be collected. The bituminous part[51] is melted out in the form of tar. There is disengaged at the same time, a large quantity of an aqueous fluid, contaminated with a portion of oil, and various ammoniacal salts. A large quantity of carburetted hidrogen, and other uninflammable gases, make their appearance, and the fixed base of the coal remains behind in the distillatory apparatus in the form of a carbonaceous substance, called coke.
All these products may be separately collected in different vessels. The carburetted hidrogen, or coal-gas, may be freed from the non-inflammable gases, and afterwards forced in streams out of small appertures, which, when lighted, may serve as the flame of a candle to illuminate a room or any other place. It is thus, that from pit-coal a native production of this country, we may procure a pure, lasting, and copious light; which, in other cases, must be derived from expensive materials, in part imported from abroad.
It is chiefly upon the power of collecting the products afforded by coal, with convenience and cheapness, that the promoters of the gas-light illumination found their claims to public encouragement. They conceive that the flame[52] which pit-coal yields, as it is now consumed, is turned to very little advantage: it is not only confined to one place, where a red heat is more wanted than a brilliant flame, but it is obscured, and sometimes entirely smothered, by the quantity of incombustible materials that ascend along with it and pollute the atmosphere.
That much inflammable matter is thus lost, is evident from facts that come under our daily observation. We often see a flame suddenly burst from the densest smoke, and as suddenly disappear; and if a light be applied to the little jets that issue from the bituminous parts of the coal, they will catch fire, and burn with a bright flame. A considerable quantity of a gazeous fluid, capable of affording light and heat continually escapes up the chimney, whilst another part is occasionally ignited, and exhibits the phenomena of the flame and light of the fire.
The theory of the production of gas-light is therefore analogous to the action of a lamp or candle. The wick of a candle being surrounded by the flame, is in the same situation of the pit-coal exposed to distillation. The office of the wick is chiefly to convey tallow, by capillary[53] attraction, to the place of combustion. As it is decomposed into carburetted hidrogen gas it is consumed and flies off, another portion succeeds; and in this way a continued current of tallow and maintenance of flame are effected. See page 15.
The combustion of oil by means of a lamp depends on similar circumstances. The tubes formed by the wick serve the same office as a retort placed in a heated furnace through which the inflammable liquid is transmitted. The oil is drawn up into these ignited tubes, and is decomposed into carburetted hidrogen gas, and from the combustion of this gas the illumination proceeds. See p. 15. What then does the gas-light system attempt? Nothing more than to generate, by means of sufficient furnaces and a reservoir of sufficient capacity, desired quantities of the gas, which is the same material of the flame of candles or lamps; and then by passing it through pipes to any desired distance, to exhibit it there at the mouths of the conducting tubes, so that it may be ignited for any desired purpose. The only difference between this process and that of an ordinary candle or lamp, consists in having the furnace[54] at the manufactory, instead of its being in the wick of the candle or lamp—in having the inflammable material distilled at the station, instead of its present exhibitions in oil, wax, or tallow, and then in transmitting the gas to any required distance, and igniting it at the orifice of the conducting pipe instead of igniting it at the apex of the wick. The principle is rational, and justified by the universal mode in which all light is produced. Indeed, this discovery ranks among the numerous recent applications of chemical science to the purposes of life, which promise to be of the most general utility.
It is evident from the outline here given of the production and application of coal-gas, that all the uses of pit-coal are not exhausted; it will be sufficient to observe, that the complete analysis of coal, which has been hitherto confined to the laboratory of the chemist, requiring skill and nicety in the operator, and attended with great trouble and expence, is now so far simplified, that many chaldrons of coals may be decomposed by one gas-light apparatus in the space of six hours, and all the component parts produced in their most useful[55] shape, at an expence out of all proportion below the value of the products.
To assist the reader in comprehending the nature and object of substituting coal-gas for tallow or oil, for the purpose of obtaining light, it may be proper to touch slightly upon the successive discoveries that have been made as to the decomposition of coal, and the application of its different ingredients. Such a sketch will add to the many examples that occur in the history of science and art, showing the slow progress of mankind in following up known principles, or extracting from acknowledged facts every possible advantage.
In the Philosophical Transactions of the[56] Royal Society, V. XLI. so long ago as the year 1739, is recorded a paper, exhibiting an account of some experiments made by Dr. James Clayton, from which it appears that the inflammable nature of coal-gas was then already known. Dr. Clayton having distilled Newcastle coal, obtained, as products of the process, an aqueous fluid, a black oil, and an inflammable gas, which he caught in bladders, and by pricking these he was enabled to inflame the gas at pleasure.
It is further known, that in the beginning of the last century, Dr. Hales[11] on submitting pit-coal to a chemical examination, found, that during the ignition of this fossil in close vessels, nearly one-third of the coal became volatilized in the form of an inflammable vapour. Hence the discovery of the inflammable nature of coal-gas can no longer be claimed by any person now living.
[11] Vegetab. Statics, vol. I.
In the year 1767, the Bishop of Llandaff[12] examined the nature of the vapour and gazeous products evolved during the distillation of pit-coal. This learned philosopher noticed, that[57] the volatile product is not only inflammable as it issues from the distillatory vessel, but that it also retained its inflammability after having been made to pass through water, and suffered to ascend through two high curved tubes. The solid matters obtained by this venerable prelate, were, an aqueous ammoniacal fluid, a tenaceous oil, resembling tar, an ammoniacal liquor, and a spongy coal, or coke.
[12] Watson’s Chemical Essays, vol. II.
The first discovery and application of the use of coal-gas for the purpose of illumination is claimed by Mr. Murdoch.
Dr. W. Henry of Manchester, has published the following account[13] of this discovery.
[13] Thompson’s System of Chemistry, vol. I. p. 52.
“In the year 1792, at which time Mr. Murdoch resided at Redruth, in Cornwall, he commenced a series of experiments upon the quantity and quality of the gases contained in different substances. In the course of these he remarked, that the gas obtained by distillation from coal, peat, wood, and other inflammable substances, burnt with great brilliancy upon being set fire to; and it occurred to him, that by confining and conducting it[58] through tubes, it might be employed as an economical substitute for lamps and candles. The distillation was performed in iron retorts, and the gas conducted through tinned iron and copper tubes to the distance of 70 feet. At this termination, as well as at intermediate points, the gas was set fire to, as it passed through apertures of different diameters and forms, purposely varied with a view of ascertaining which would answer best. In some the gas issued through a number of small holes like the head of a watering pan; in others it was thrown out in thin long sheets; and again in others in circular ones, upon the principle of Argand’s lamp. Bags of leather and of varnished silk, bladders, and vessels of tinned iron, were filled with the gas, which was set fire to, and carried about from room to room, with a view of ascertaining how far it could be made to answer the purpose of a moveable or transferable light. Trials were likewise made of the different quantities and qualities of gas produced by coals of various descriptions, such as the Swansea, Haverfordwest, Newcastle, Shropshire, Staffordshire, and some kinds of Scotch coals.
“Mr. Murdoch’s constant occupations prevented his giving farther attention to the subject at that time; but he again availed himself of a moment of leisure to repeat his experiments upon coal and peat at Old Cumnock, in Ayrshire, in 1797; and it may be proper to notice that both these, and the former ones, were exhibited to numerous spectators, who, if necessary, can attest them. In 1798, he constructed an apparatus at Soho Foundry, which was applied during many successive nights to the lighting of the building; when the experiments upon different apertures were repeated and extended upon a large scale. Various methods were also practised of washing and purifying the air, to get rid of the smoke and smell. These experiments were continued, with occasional interruptions, until the epoch of the peace in the spring of 1802, when the illumination of the Soho manufactory afforded an opportunity of making a public display of the new lights; and they were made to constitute a principal feature in that exhibition.”
In the year 1803 and 1804, Mr. Winsor exhibited at the Lyceum in London the general[60] nature of this new mode of illumination though the machinery for procuring, and the manner of purifying the gas, he kept a secret. He exhibited the mode of conducting the gas through the house, and a number of devices for chandeliers, lamps, and burners, by which it might be applied. Among these he proposed long flexible tubes suspended from the ceiling, or wall of the room, and at the end communicating with burners or lamps of different kinds. This gentleman showed also by experiment, that the flame of the gas-light, produced no smoke; that it was not so dangerous as the flame of candles or lamps; that it could not produce sparks; and that it was not so readily extinguished by gusts of wind or torrents of rain.
Mr. Winsor’s display of gas-lights took place more than two years before Mr. Murdoch’s priority of right was heard of.
In stating these facts I do not mean to say that Mr. Murdoch derived the hint of applying the coal-gas from the previous exhibition of Mr. Winsor, because it is quite within the bounds of probability that the ideas of Mr.[61] Murdoch may have arisen totally independent of all acquaintance with Mr. Winsor’s.
The claims of invention, or the determination of the right of priority, concerns the public only so far as the honour and estimation of any useful discovery conferred on the inventor may induce other individuals to devote their talents to similar pursuits; by means of which, more discoveries may be made, and the subject of human invention become extended, or rendered more useful. For as the mere benefits which mankind may derive from any particular discovery, they are certainly more indebted to the person who first applied the discovery to actual practice, than to him who first made it, and merely illustrated it by barren experiments. Mr. Winsor certainly pressed on the mind of the public with unremitted perseverance and diligence the extensive application of gas-light in the year 1802, but he made no new discovery with regard to the composition of coal; he did not even invent the mode of conducting the gas through tubes; and if he has pointed out the particulars of the process, he has made a very important, though not the most brilliant improvement in this line of business. Mr.[62] Winsor’s publications are, perhaps, but ill adapted to promote his cause; and the exaggerated calculation which the sanguine mind of a discoverer is naturally disposed to indulge in, have, to superficial observers, thrown an air of ridicule and improbability on the whole scheme of lighting with gas.
It may, however, be safely affirmed, that if the same facts had come forward, under the sanction of some great name in the chemical or philosophical world, the public incredulity would long since have been subdued; and the plan, which for many years has been struggling for existence, would have been eagerly adopted as a national object.
On the 18th of May, 1804, Mr. Frederick Albert Winsor, took out a patent for combining the saving and purifying of the inflammable gas (for producing light and heat), the ammonia, tar, and other products of pit-coal, with the manufacture of a superior kind of coke (see Repertory, 2d Series, v. 172). And, lately, the same gentleman has taken out a second patent, for further improvements in these processes.
In the year 1805, Mr. Northern, of Leeds,[63] also directed the attention of the public to the application of coal-gas, as a substitute for tallow light, as will be seen by the following extract of the Monthly Magazine for April, 1805.
“I distilled in a retort, 50 ounces of pit-coal in a red heat, which gave 6 ounces of a liquid matter covered with oil, more or less fluid as the heat was increased or diminished. About 26 ounces of cinder remained in the retort; the rest came over in the form of air, as it was collected in the pneumatic apparatus. I mixed part of it with atmospherical air, and fired it with the electric spark with a tolerable explosion, which proves it to be hydrogene.—Whether any of the other gases were mixed with it, I did not then determine. In the receiver I found a fluid of an acid taste, with a great quantity of oil, and, at the bottom, a substance resembling tar.
“The apparatus I make use of for producing light is a refiner’s crucible, the top of which (after filling with coal) I close with a metal cover, luted with clay or other luting, so as to prevent the escape of the gas; a metal pipe is soldered into the cover, bent so as to[64] come under the shelf in the pneumatic trough, over which I place a jar with a stop-cock and a small tube; the jar being previously filled with water, the crucible I place on the common or other fire as is most convenient; and as the heat increases in it, the gas is forced rapidly through the water into the jar, and regularly displaces it. I then open the cock and put fire to the gas, which makes its escape through the small tube, and immediately a most beautiful flame ensues, perfectly free from smoke or smell of any kind. A larger light, but not so vivid or clear, will be produced without passing the gas through water, but attended with a smoke somewhat greater than that of a lamp charged with common oil.
“I have great hopes that some active mechanic or chemist will, in the end, hit on a plan to produce light for large factories, and other purposes, at a much less expence, by the above or similar means, than is at present produced from oil.”
Soon afterwards, Mr. Samuel Clegg[14] of Manchester, Engineer, communicated an account of his method of lighting up manufactories[65] with gas-light to the Society of Arts, for which he received the silver medal.
[14] This gentleman is at present engineer to the Gas-Light Company.
Since that time, the application of gas-light has spread rapidly, and numerous manufactories and other establishments have been lighted by coal-gas.
In France, the application of gas-lights to economical purposes, was pointed out long before it was publicly introduced into this country. M. Le Bon had a house fitted up in Paris, in the winter of 1802, so as to be entirely illuminated by gas-lights, which was seen by thousands with admiration; and had a brevet d’invention (patent) granted to him by the French government, for the art of producing light from wood, ignited in close vessels.
Many other attempts have been made to derive advantage from the different ingredients of coal; but they are too obscure to merit particular enumeration.
In the year 1808, Mr. Murdoch presented to the Royal Society his account of the application of gas-light, and was complimented with Count Romford’s medal for the same.
The following statement is taken from Mr. Murdoch’s paper.
“The whole of the rooms of the cotton mill of Mr. Lee, at Manchester, which is I believe the most extensive in the United Kingdom, as well as its counting-houses and store-rooms, and the adjacent dwelling house of Mr. Lee, are lighted with the gas from coal. The total quantity of light used during the hours of burning has been ascertained, by a comparison of shadows, (see page 23) to be about equal to the light which 2500 mould candles, of six to the pound, would give; each of the candles with which the comparison was made consuming at the rate of 4-10ths of an ounce (175 grains) of tallow per hour.
“The gas-burners are of two kinds: the one is upon the principle of the Argand lamp, and resembles it in appearance; the other is a small curved tube with a conical end, having three circular apertures or perforations, of about a thirtieth of an inch in diameter, one at the point of the cone, and two lateral ones, through which the gas issues, forming three divergent jets of flame, somewhat like a fleur-de-lis. The shape and general appearance of this tube has procured it, among the workmen, the name of the cockspur burner.
“The number of burners employed in all the buildings amounts to 271 Argand, and 653 cockspurs, each of the former giving a light equal to that of four candles of the description above-mentioned; and each of the latter a light equal to two and a quarter of the same candles; making therefore the total of the gas-light a little more than equal to that of 2500 candles, six to the pound. When thus regulated, the whole of the above burners require an hourly supply of 1250 cubic feet of the gas produced from cannel-coal; the superior quality and quantity of the gas produced from that material having given it a decided preference in this situation over every other coal, notwithstanding its higher price.
“The time during which the gas-light is used may, upon an average of the whole year, be stated at least at two hours per day of 24 hours. In some mills, where there is over work, it will be three hours; and in the few where night work is still continued nearly 12 hours. But taking two hours per day as the common average throughout the year, the consumption in Messrs. Philips and Lee’s mill will be 1250 × 2 = 2500 cubic feet of gas per day; to produce which 700 weight of[68] cannel-coal is required in the retort. The price of the best Wiggan cannel-coal (the sort used) is 131⁄2d. per cwt. (22s. 6d. per ton) delivered at the mill, or say about eight shillings for the seven hundred weight. Multiplying by the number of working days in the year (313,) the annual consumption of coal will be 110 tons, and its cost 125l.
“About one-third of the above quantity, or say forty tons of good common coal, value ten shillings per ton, is required for fuel to heat the retorts, the annual amount of which is 20l.
“The 110 tons of cannel-coal, when distilled, produce about 70 tons of good coke, which is sold upon the spot at 1s. 4d. per cwt. and will therefore amount annually to the sum of 93l.
“The quantity of tar produced from each ton of cannel-coal is from 11 to 12 ale gallons, making a total annual produce of about 1250 ale gallons, which not having been yet sold, it cannot yet be determined its value.
“The interest of the capital expended in the necessary apparatus and buildings, together with what is considered as an ample allowance for wear and tear, is stated by Mr. Lee at about 550l. per annum, in which some allowance is[69] made for this apparatus being made upon a scale adequate to the supply of a still greater quantity of light, than he has occasion to make use of.
“Mr. Lee is of opinion that the cost of attendance upon candles would be as much, if not more, than upon the gas apparatus; so that, in forming the comparison, nothing need be stated upon that score, on either side.
“The economical statement for one year, then, stands thus:
| Cost of 110 tons of cannel coal | £ 125 |
| Ditto of 40 tons of common ditto, to carbonise | 20 |
| In all | 145 |
| Deduct the value of 70 tons of coke | 93 |
| The annual expenditure in coal, after deducting the value of the coke, and without allowing any thing for the tar, is therefore | 52 |
| And the interest of capital sunk, and wear and tear of apparatus | 550 |
| Making the total expence of the gas apparatus per annum, about | 600 |
“That of candles, to give the same light,[70] would be about 2000l. For each candle, consuming at the rate of 4-10ths of an ounce of tallow per hour, the 2500 candles burning, upon an average of the year, two hours per day, would, at one shilling per pound, the present price, amount to nearly the sum of money above-mentioned.
“If the comparison were made upon an average of three hours per day, as in most cases, would perhaps be nearer to the truth, and the tear and wear remaining nearly the same as on the former case, the whole cost would not exceed 650l. while that of the tallow would be 3000l.”
Mr. Ackerman in this metropolis, has shown that the art of gas-light illumination is not confined to great manufactories, but that its advantages are equally applicable to those on a moderate scale. The whole of Mr. Ackerman’s establishment, his public library, warehouse, printing-offices and work-shops, together with his dwelling house, from the kitchen to the drawing-room, has, for these four years past, been lighted with gas, to the total exclusion of all other lights. The result of the whole of this proceeding will be obvious from the following letter:
To Mr. ACCUM.
Sir,
“In answer to your request with regard to my gas-lights, which I now have in my house, I take this mode of informing you, that I charge two retorts with 240lbs. of coal, half cannel and half Newcastle, from which I extract 1000 cubic feet of gas. To obtain this quantity of gas, when the retorts are cold, I use from 100 to 110lb. of common coals; but when they are in a working state, that is to say, when they are once red hot, the carbonising fuel amounts to about 25lb. per retort. The bulk of gas thus obtained supplies 40 Argand’s lamps, of the large size, for four hours per night, during the long winter evenings, together with eight Argand’s lamps and about 22 single cockspur burners, for three hours per night: in addition to which my printers employ 16 cockspur burners for ten hours per day to heat their plates instead of charcoal fire. In the depth of winter we charge two retorts per day: but, upon an average, we work 365 retorts in 365 days.
Now 365 retorts containing 120lb. of coal each, make 43800lb. which is equal to ten chaldrons of Newcastle and eight tons of cannel coal.
10 chaldrons of Newcastle coals, at 65s. make £ 32 10 0 8 tons of cannel coal,[15] (this coal is sold by weight) at 100s. per ton 40 0 0 7 chaldrons of common coals for carbonising, at 55s. 19 5 0 To wages paid the servant for attending the gas apparatus 30 0 0 Interest of money sunk 30 0 0 The wear and tear of the gas-light apparatus I consider to be equal to the wear and tear of lamps, candlesticks, &c. employed for oil, tallow, &c. Total expence of the gas lights 151 15 0 DEDUCT 23 chaldrons of coke, at 60s. per chaldron 69 Ammoniacal liquor 5 Tar 6 Charcoal employed by the copper-plate printers to heat their plates, which is now done with the gas-light flame, cost, annually 25 Two chaldrons of coals minus used as fuel, for warming the house, since the adoption of the gas-lights, at 65s. per chaldron 6 10 111 10 0 Nett expences of the gas-lights £ 40 5 0 The lights used in my Establishment, prior to the gas-lights, amounted annually to 160 0 0 My present system of lighting with gas costs, per ann. 40 5 0 Balance in favor of the gas for one year £ 119 15 0
[15] Although cannel-coal sells at nearly double the price of Newcastle coal, I use it in preference to the latter, because it affords a larger portion of gas, and gives a much more brilliant light.
Such is the simple statement of my present system of lighting, the brilliancy of which, when contrasted with our former lights, bears the same comparison to them as a bright summer sun-shine does to a murky November day: nor are we, as formerly, almost suffocated with the effluvia of charcoal and fumes of candles and lamps. In addition to this, the damage sustained by the spilling of oil and tallow upon prints, drawings, books and paper, &c. amounted annually to upwards of 50l. All the workmen employed in my establishment consider their gas-lights as the greatest blessing; and I have only to add, that the light we now enjoy, were it to be produced by means of Argand’s lamps or candles, would cost at least 350l. per annum.
I am, with respect,
Yours,Strand, March 13,
1815.R. ACKERMAN.”
Another manufacturer who was one of the first that adopted the use of this method of illumination in the small way, and who gave a statement of its advantages to the public, is Mr. Cook, a manufacturer of metal toys, at Birmingham, a clear-headed, prudent man, not apt to be dazzled by a fanciful speculation, but governed in his transactions by a simple balance of profit and loss. There is a naïveté in his own account of the process which will amuse as well as instruct the reader.
“My apparatus is simply a small cast-iron pot, of about eight gallons, with a cast-iron cover, which I lute to it with sand. Into this pot I put my coal. I pass the gas through water into the gasometer or reservoir, which holds about 400 gallons; and, by means of old gun-barrels, convey it all round my shops. Now, from twenty or twenty-five pounds of coal, I[73] make perhaps six hundred gallons[16] of gas; for, when my reservoir is full, we are forced to burn away the overplus in waste, unless we have work to use it as it is made: but, in general, we go on making and using it, so that I cannot tell to fifty or a hundred gallons;—and, in fact, a great deal depends on the coals, some coals making much more than others. These twenty-five pounds of coal put into the retort, and say twenty-five pounds more to heat the retort, which is more than it does take one time with another, but I am willing to say the utmost, are worth four-pence per day. From this four-pence we burn eighteen or twenty lights during the winter season.”
[16] A wine-gallon is equal to 231 cubic inches.
Thus are the candles which Mr. Cook used to employ, and which cost him three shillings a day, entirely superseded. But, besides his expence in candles, oil and cotton for soldering, used to cost him full 30l. a year; which is entirely saved, as he now does all his soldering by the gas flame only. For “in all trades in which the blow-pipe is used with oil[74] and cotton, or where charcoal is employed to produce a moderate heat, the gas flame will be found much superior, both as to quickness and neatness in the work: the flame is sharper, and is constantly ready for use; while, with oil and cotton or charcoal, the workman is always obliged to wait for his lamp or coal getting up; that is, till it is sufficiently on fire to do his work. Thus, a great quantity of oil is always burned away useless; but, with the gas, the moment the stop-cock is turned, the lamp is ready, and not a moment is lost.” We must refer to Mr. Cook’s letter for the details of expence, which he gives with faithful minuteness, and always leaning to the side unfavourable to the gas. The result of the whole is, that he saves 30l. out of the 50l. which his lights formerly cost him: and, when we consider that his calculation allows the gas-lights to burn the whole year, and the candles only twenty weeks, there can be little doubt, that the savings in this case follow nearly the same proportion as in the former. If the apparatus be erected even on a smaller scale, “the saving,” Mr. Cook assures us, “will still be considerable:[75] for the poor man, who lights only six candles, or uses one lamp, if the apparatus is put up in the cheapest way possible, will find it only cost him 10l. or 12l. which he will nearly, if not quite, save the first year.”
Mr. Ackerman having, in this town, set the example of lighting his establishment with gas, several other individuals soon followed the attempt. The following statement will show, that this species of light may be made use of with the greatest advantage, upon a still smaller scale, where no great nicety with regard to the apparatus for procuring gas is required. The following report I have received from Messrs. Lloyd, of Queen Street, Southwark, thimble manufacturers and whitesmiths, who have used the gas-light for soldering and other purposes these five years past.
| From 4 pecks or 1 bushel of coals, weighing 69lbs. for which we now pay (1809) 1s. we produce 43⁄4 pecks of coke and 1⁄2 peck of coal not carbonised remains in the distilling pot, which together with the coke weighs 58lbs. 6 oz. value at 1s. per bushel | 0 | 1 | 4 |
| we procure 6lbs. 4 oz. of tar which[76] we use as pith—it saves us | 0 | 1 | 0 |
| 0 | 2 | 4 | |
| Deduct for coal | 0 | 1 | 0 |
| Profit on coke and tar | 0 | 1 | 0 |
| The gas yielded by the 4 pecks of coals in the pot, make 42 brilliant lights, which burn 7 hours. To keep 42 tallow candles which were formerly used in the manufactory burning for the same time, required 7lbs. which at 1s. per lb. cost | 0 | 7 | 0 |
| To this, add profits on coke and tar | 0 | 1 | 0 |
| Gained out of every bushel of coal | 0 | 8 | 0 |
“The gas-burners made use of in our manufactory produce jets of flame, which in our business, where much soldering with the blow-pipe must be done, have a decided superiority over Argand’s lamps. We are not nice concerning the quality of the gas—a great part of it is burned from the gasometer, without allowing it to purify itself in the gasometer, because our gasometer is not large enough to store up the whole quantity of gas we want for use.”
To obtain carburetted hidrogen, or coal-gas, from common pit-coal, and to apply it for the purposes of illumination, the coal is introduced into large iron cylinders, called retorts, to the apertures of which iron pipes are adapted, terminating in a vessel, or vessels, destined to purify and collect the gas. The retorts charged with coals and made air-tight, are placed upon the fire, the action of which extricates the gazeous products from the coals, together with an aqueous ammoniacal vapour, and a tenaceous bituminous fluid, or tar, &c. The liquid substances are conveyed into proper vessels, and the gazeous products are conducted, by means of pipes, under the gasometer, where the[78] gas is again washed, and remains ready for use. There are also other pipes leading from the gasometer, which branch out into smaller ramifications, until they terminate at the places where the lights are wanted. The extremities of the pipes have small apertures, out of which the gas issues, and the streams of gas being lighted at those apertures burn with a clear and steady flame as long as the supply of gas continues. All the pipes which come from the gasometer are furnished at their extremities with stop-cocks to regulate the admission of the gas. The burners are formed in various ways, either a tube ending with a simple orifice, at which the gas issues in a stream, and if once lighted will continue to burn with the most steady and regular light imaginable, as long as the gas is supplied; or two concentric tubes of brass, or sheet-iron, are placed at a distance of a small fraction of an inch from each other, and closed at the bottom. The gas which enters between these cylinders, when lighted, forms an Argand lamp, which is supplied by an internal and external current of air in the usual manner. Or the two concentric tubes are closed at the top with a ring having small perforations, out of[79] which the gas alone can issue, thus forming small distinct streams of light.
The gas-apparatus, plate 2, will be found very convenient for exhibiting, in the small way, the general nature of this new art of illumination, whilst at the same time it may serve to ascertain, at a trifling expence, the comparative value of different kinds of coals intended to be employed for the production of this species of light, as well as other occasional purposes connected with the gas-light system of illumination.
It consists of three distinct apparatus:—namely, a portable furnace, fig. 1, plate 2, by means of which the gas is prepared—fig. 2, a purifyer, or condenser, which separates and purifies the products obtained from the coal, so as to render the gas fit for the purpose of illumination—fig. 3, a gasometer, or reservoir for receiving and preserving the purified stock of gas, and from which it may be transferred and distributed as occasion may require. The following statement will explain more fully the general nature of this portable chamber apparatus:—a, represents a cast iron retort, such as is used for chemical operations in the small[80] way. This retort rests upon a tripod of hammered iron, placed upon the bars of the grate of the chemical furnace. Into this retort the coals are put for furnishing the gas. It is provided with a solid iron stopper ground air-tight into the mouth of the retort, and the stopper is secured in its place by an iron wedge passing over it in the centre; by means of which the mouth of the retort when charged with coal is readily made air-tight, and the stopper may easily be removed by knocking out the iron wedge. b. is a metal pipe which conveys all the distillatory products from the retort into the purifier fig. 2. This tube is bent at right angles at the extremity where it enters the intermediate vessel fig. 2. The purifier fig. 2, is divided into three compartments marked c. d. e. The first compartment is filled with water, and by means of it an air-tight communication is established with the retort which furnishes the gas. The second compartment, d, contains a solution of caustic pot-ash composed of about 2 parts of caustic pot-ash and 16 of water, or a mixture of quick-lime and water of the consistence of very thin cream. The object of this compartment is to separate the non-inflammable[81] gases and other products evolved during the distillation of the coal, from the carburetted hidrogen or coal-gas, so as to render it fit for use. The third compartment e is left empty to receive the tar and other liquid products. Into the first compartment c, all the gazeous and liquid products are delivered, as they become evolved during the distillation, by means of the pipe b. The compartment d, of the purifier, or alcali vessel, is furnished with a wide perpendicular pipe, which serves to make an air-tight communication with the retort, by allowing the tube b, to pass readily through it. From the chamber c, the liquid and gazeous products pass to the tar-chamber, or compartment e, by means of the descending pipe f. The tar and other condensible substances are therefore deposited at e, whilst the gazeous products alone ascend from the tar-chamber e, by the pipe g, and down again the pipe h, (which is closed at the top) into the compartment d, of the vessel or purifier, fig. 2. The gas being thus made to pass from the compartment e, up into the pipe g, and down the pipe h, (which is closed at the top) into the purifier d, is brought into contact with[82] the liquor in that vessel, where it is opposed to a pressure in proportion to the perpendicular height of the column of liquid which it contains. The funnel in the compartment c, is considerably higher than the purifying apparatus, it therefore allows the liquid which it contains, when pressed upon by the gas, to ascend into it, without overflowing the apparatus, and to descend again as the pressure diminishes—i is another wide-mouth funnel, by means of which the chamber d, is filled with the alcaline solution, or mixture of lime and water. The carbonic acid gas and sulphuretted hidrogen, evolved during the distillation of the coal, are thus made to combine with the alcali or lime, in the compartment d, of the purifier, forming carbonate and hidro-sulphuret of lime. The carburetted hidrogen, being left more or less pure, is conveyed through the pipe k, into the gasometer, fig. 3. The communication of the purifier, fig. 2, with the gasometer, is made by means of the well-known water-valve l, placed so that the communicating tube k, may be easily removed at pleasure—m, is a cock for drawing off the tar, &c. n, a gauge-cock for ascertaining the height of[83] the liquid in the chamber d. The gasometer, fig. 3, the object of which is to store up the gas, consists of two principal parts—namely, a large interior vessel designed to contain the gas, and an outer cistern or vessel, of rather greater capacity, in which the former is suspended, designed to contain the water by which the gas is confined. The interior vessel which contains the gas is suspended by chains or cords hung over pullies, to which weights are attached, so as to nearly equipoise it. o is a pipe, which communicates with the water-valve l, and by means of which the gas passes from the purifier, fig. 2, into the gasometer. The upper end of this pipe is covered, in the manner of a hood, by a cylindrical vessel p, open at bottom, but partially immersed beneath the surface of the water contained in the outer cistern of the gasometer, and perforated round near the lower edge with a number of small holes. The gas displaces the water from this receiver p, and escapes through the small holes, rising in bubbles through the water, so as to expose a large surface to its action, that it may be properly washed, &c. After rising through the water the gas enters[84] the gasometer, which is suspended to move up and down by the chains, pullies, and balance-weights, q. From the centre of the gasometer a tube, r, descends, which includes a pipe, s, fixed perpendicular from the bottom of the cistern. The fixed pipe r, forms a guide to keep the gasometer always perpendicular. t is also an iron pipe made fast in the centre of the inner vessel, and communicates with the upright tube, s, in the outer vessel. This contrivance obliges the gas to pass into the pipe t, whilst it also serves to keep the gasometer steady when nearly out of the outer cistern.
When the operation commences, the gasometer is sunk down nearly to a level with the surface of the water in the outer cistern, and is consequently filled with water; but as the gas enters, it rises up to receive it. It is to be noted, that the balance-weights q q, should not be quite so heavy as the gasometer, in order that some pressure may be exerted, to force the gas out of the burners with a proper jet. The gas which issues from the retort enters the purifier as stated already, and ascends the pipe o, into the vessel, p, from which it displaces the water, and passes out at the small holes, as[85] before described, rising through the water into the gasometer, and raising it up: the gas then passes away to the burners, u u. In this manner the process proceeds until the whole of the volatile products of the coal in the retort is evaporated. The use of the gasometer is, to equalize the emission of the gas which comes from the retort more quickly at some time than others. When this happens, the interior vessel rises up to receive it, and when the stream from the retort diminishes, the weight of the gasometer expels its contents. When the process is finished, the retort is suffered to cool, and its ground stopper is then removed to replenish it with coal. The residue found in the retort is coke. v v are cocks to let off any liquid that may collect in the pipe o or t; for if the smallest portion of liquid were to obstruct the free passage of the gas to the burners, the consequence would be, that the lights would not burn steadily—they would, as it is called, dance, or become extinguished. x is the main stop-cock which communicates with the burners—these, of course, may be placed as convenience may require. z z are two projecting parts in the top of the gasometer;[86] they are intended to receive the hood p, and the upper extremity of the pipe t, so as to allow the gasometer to be wholly immersed into the cistern. The wheels or pullies of the gasometer have a groove to allow the links of the chain to pass freely.
In this apparatus there is no provision made for the unequal pressure which the gas suffers, accordingly as the gasometer is more or less immersed in water. It will be observed that, in this apparatus, the weight of the interior vessel is constantly increasing, in proportion as it fills with gas, and rises out of the water, and consequently, if a constant, uniform, counterpoising weight, equal only to that of the gasometer in the first moment of its rise, be employed, the gas becomes gradually more and more compressed by that part of the weight of the gasometer which is not counterpoised, and if its pressure or quantity be then estimated by the bulk which it occupies, without making allowance for the increasing pressure, a material error must arise, and this, in the large way, would give rise to insurmountable difficulties with regard to the regulation[87] of the size of the flames; which could not be rendered uniform.
Suppose the cistern or exterior vessel full of water, and the gasometer partly filled with gas and partly with water, it is evident that the balance-weight may be so adjusted, as to occasion an exact equilibrium, so that the external air shall not tend to enter into the gasometer nor the gas to escape from it; and in this case the water will stand exactly at the same level both within the gasometer and within the outer cistern. On the contrary, if the balance-weights be diminished, the gasometer will then press downwards from its own gravity, and the water will stand lower in the gasometer than it does in the cistern; in this case, the included air or gas will suffer a degree of compression above that experienced by the external air, exactly proportioned to the weight of a column of water, equal to the difference of the external and internal surfaces of the water.
To compensate for this increasing weight of the gasometer, and render a scale of equal graduations accurate, some have ingeniously[88] adopted the plan of a spiral pulley to the chain, which has the effect of gradually avoiding the evil, but the best way of accomplishing it will be stated hereafter.
With regard to the philosophy or the production of coal-gas, it proves that pit-coal contains solid hidrogen, carbon, and oxigen. When the intensity of the heat has reached a certain degree, a part of the carbon unites with part of the oxigen and produces carbonic acid, which by means of caloric is melted into the gazeous state and forms carbonic acid gas; at the same time, part of the hidrogen of the coal combines with another portion of carbon and caloric, and forms the carburetted hidrogen gas, which varies considerably in its constitution, according to the circumstances under which it is produced; a portion of olifiant gas, carbonic oxid, hidrogen, and sulphuretted hidrogen, is also produced during the process. The quantities of these products vary according to the nature of the coal employed in the process.
Pit-coal is not the only substance which affords carburetted hydrogen; this gazeous fluid[89] may be obtained in a great variety of ways, and with very considerable differences in specific gravity and proportion of ingredients.
It is found plentifully native or ready formed on the surface of stagnant waters, marshes, wet ditches, &c. through which, if examined closely, large bubbles will be seen to rise in hot weather, and may be increased at pleasure by stirring the bottom or mud with a stick.
In close still evenings if a lighted candle is held over the surface, flashes of blue lambent flame may sometimes be perceived spreading to a considerable distance. All that is not fabulous concerning the ignis fatuus is probably derived from this source. This species of gas is termed for distinction the carburetted hydrogen of marshes. In the purest form in which it can be collected it is mixed with about 20 per cent. of azot or nitrogen.
To procure the gas for the purpose of philosophical amusement, fill a wide-mouthed bottle with the water of the ditch, and keep it inverted therein with a large funnel in its neck, then with a stick stir the mud at the bottom just under the funnel, so as to cause the bubbles of air which rise from the mud to enter[90] into the bottle; when by thus stirring the mud in various places, the air may be catched in the bottle.
Carburetted hidrogen gas is also given out very abundantly by all kinds of vegetable matter when subjected to a scorching heat sufficient to decompose them. When heated in close vessels much more gas is obtained than when burnt in the open air. If moistened charcoal be put into an earthen retort and heat be applied till the retort becomes ignited; gas will be evolved, consisting partly of carbonic acid, and partly of carburetted hidrogen. A gas of similar properties is obtained by causing steam to pass through a tube filled with red-hot charcoal; by passing spirit of wine, or camphor, through red-hot tubes; by distilling oils, wood, bones, wax and tallow, or any animal or vegetable body whatever.
Indeed it would be endless to enumerate the various sources of this gazeous fluid. A most curious variety of carburetted hidrogen gas has been discovered by the associated Dutch chemists (Van Dieman, Troostwyck, and others) which is procured from ether or alcohol, and has the remarkable property of generating a heavy oil when in contact with chlorine gas.[91] Hence it has been termed oily carburetted hidrogen, or olifiant gas—it consists of carburetted hydrogen, supersaturated with carbon. The oil generated is heavier than water, whitish, and semi-transparent. By keeping, it becomes yellow and limpid; its smell is highly fragrant and penetrating—its taste somewhat sweet—it is partly soluble in water, imparting to it, its peculiar smell. A portion of this gas always accompanies the common carburetted hidrogen obtained from coal, and those sorts of coal that afford the largest quantity of it are best suited for the production of gas-light.
The nature of carburetted hidrogen obtained from coal varies considerably according to the conditions under which it is obtained. The first part is always much heavier than the last, though still lighter than common air, and holds in solution a portion of oil, for on standing for some time over water it becomes lighter, and is found to require less oxygen for saturation than before. The oil which it held suspended, then becomes precipitated. The average specific gravity of the first and last gas mixed, which may be taken as an average of the whole specific gravity is to that of common[92] air as 2 to 3—112lb. of common cannel coal produce at its minimum, from 350 to 360 cubic feet of carburetted hidrogen gas; but the same quantity of the best Newcastle coal, that is to say, such as coke, which, when laid on the fire readily undergoes a kind of semi-fusion, and sends out brilliant streams of flame, produces upon an average from 300 to 360 cubic feet of this gazeous fluid, besides a large portion of sulphuretted hidrogen, carbonic oxid and carbonic acid. Half a cubic foot of this carburetted hidrogen, fresh prepared, that is to say, holding in solution or suspension, a portion of the essential oil, which is generated during the evolution of the gas, is equal in illuminating power to from 170 to 180 grains of tallow, (being the quantity consumed by a candle six to the pound in one hour.) Now, one pound avoirdupoise is equal to 7000 grains, and consequently one pound of candles of six in the pound, burning one at a time in succession, would last (if we take 175 grains of tallow to be consumed in an hour) 7000175 = 40 hours. To produce the same light we must burn one half of a cubic foot of coal-gas per hour; therefore, one-half multiplied by forty hours is equal to twenty cubic feet of gas in 40 hours,[93] consequently equal to one pound of candles, six to the pound, provided they were burnt one after another. One hundred and twelve pounds of cannel-coal, produce, at its minimum, three hundred and fifty cubic feet of gas; and are equal to three hundred and fifty, divided by twenty, which last is equivalent to one pound of tallow, making one hundred and twelve pounds of cannel-coal, equal to 35020 = 171⁄2lbs. of tallow. Further, one hundred and twelve pounds of cannel-coal, divided by seventeen and a half of tallow make six and four-tenths of cannel-coal, equal to one pound of tallow.
With regard to Newcastle coals[17], it may be stated that one chaldron of Wall’s-End coal may be made to produce in the large way upwards of 11,000 cubic feet of crude gas; which, when properly purified, diminishes to nearly 10,000 cubic feet.
[17] One chaldron of Newcastle coal weighs from 2850 to upwards of 2978lb.
The production of carburetted hydrogen, both with regard to quantity and quality from the same kind of coal depends much upon the degree of temperature employed in the distillatory process. If the tar and oil produced during[94] the evolution of the gas in its nascent state, be made to come in contact with the sides of the red hot retorts, or if it be made to pass through an iron cylinder or other vessel heated red hot, a large portion becomes decomposed into carburetted hydrogen gas and olifiant gas, and thus a much larger quantity of gas is produced than would be obtained without such precaution from the same quantity of coal.[18]
[18] One pound of coal-tar produces 15 cubic feet of carburetted hidrogen abounding in olifiant gas.
The distillation of the coal, (if gas be the chief object) should therefore not be carried on too rapidly. Most of the retorts used in the large way, are calculated for containing about one hundred weight of coal, and in general, when previously heated, produce from two and one-half to three cubic feet of gas, in four hours for each pound of coal they contain; but when the layer of coals in them does not exceed four inches in depth, three and one-half to four feet of gas may be obtained in the same time.
The retorts best calculated for large gas-light works are seven or eight foot long (without the mouth-piece) and twelve inches in diameter,[95] tapering down to ten inches—if they are larger the coal which they contain cannot be heated properly. The advantages that may be derived from the circumstances before stated are of greater value in the gas-light manufacture than is often imagined, and the quantity as well as the quality of the gas is very much influenced by such circumstances. If coal be distilled with a very low red heat scarcely observable by daylight, the gas produced gives a feeble light—if the temperature be increased so that the distillatory vessel is of a dull redness, the light is more brilliant and of a better colour—if a bright or cherry-red heat be employed the gas produced, burns with a brilliant white flame, and if the heat be increased so far that the retort is almost white hot, and consequently in danger of melting, the gas given out, has little illuminating power, and burns with a clear blueish flame;[19] or if the coal abounds in pyrites or sulphuret of iron, as is sometimes the case with Newcastle coal, a large quantity of sulphuretted hidrogen is likewise evolved, which although it increases the illuminating power of the coal-gas, has the capital disadvantage, of producing[96] an intolerable suffocating odour, when the gas is burnt which is particularly perceptible in low rooms illuminated with such gas.
[19] It is chiefly a mixture of carbonic oxid, and hydrogen gas.
These observations also apply to the distillation of tar, which when distilled either in a vaporous or nascent state, during its first production from coal in the ordinary process, or if it be submitted to a second distillation, mingled with a fresh portion of pit-coal, a practice usually had recourse to when this product cannot be disposed of more advantageously. The best depth of coal in the retort for procuring excellent gas, and at the same time for yielding the greatest quantity from the same weight in the shortest possible time, is about six inches.
The brightness of the coal-gas flame is rather diminished when the gas has been long kept over water, and hence for illumination it should be used as soon as prepared, but of course properly purified.
The quantity of gas taken up by water is affected by temperature, because the temperature increases its elasticity; the quantity of gas absorbed, diminishes as the temperature increases, and increases as the temperature diminishes. 1⁄27 part of its own bulk of pure coal-gas[97] is absorbed by the water over which it is confined in the gazometer.
The chemical constitution of this gazeous fluid is best ascertained by burning it in a vessel of oxygen gas, over lime-water in a pneumatic reservoir, by means of a bladder and bent brass pipe. Two products are then obtained, viz. water and carbonic acid. That water is produced, may be shown by burning a very small stream of the gas in a long funnel-shaped tube open at both ends. The formation of carbonic acid is evinced, by the copious precipitation of the lime-water in the foregoing experiment.
If carburetted hydrogen be mixed with a sufficient quantity of oxygen gas or common air and fired by the electric spark, or by any other method, an explosion takes place more or less violent according to the quantity of carbonaceous matter condensed in the hydrocarbonat; and the remaining gas consists of carbonic acid, together with any unconsumed gas, or excess of oxygen, whilst the water condenses in drops on the sides of the vessel. A few cubic inches of the mixed airs is as much as can be conveniently managed at a single explosion; and when any portion of[98] olefiant gas is present, even this quantity will endanger very thick glass jars. A very vivid red flame appears at the moment of the explosion, and a great enlargement takes place in an instant, after which the bulk is suddenly reduced to much less than the original quantity. When the carbonic acid is absorbed by lime-water, if the gasses have been properly proportioned, no gazeous residue is left, except accidental impurities. Though carburetted hydrogen gas, is sometimes naturally produced in coal-mines, and occasionally mixes with common air, producing dreadful explosions, yet when coal-gas is mixed with common air, it does not explode unless the gas be to the air as 1 to 10 nearly. Such are the leading chemical habitudes of this gazeous product. The varieties of carburetted hydrogen gas all agree in being inflammable; but they possess this property in various degrees, as is evinced by the variable brightness of the flame which they yield when set on fire.
“Messrs. Sobolewsky and Horrer, of St. Petersburgh, have employed wood for the purpose of producing carburetted hydrogen gas. The pyroligneous acid obtained in this operation, when freed from the empyreumatic oil with[99] which it is mixed, becomes acetous acid, and is applicable to all the uses of vinegar. A cubic cord of wood equal to 2.133 French metres (a metre being rather more than an English yard), yields 255 Paris pounds of charcoal, and 70 buckets of acid. The latter gives 30 pounds of tar, after the extraction of it 50 buckets of good vinegar remain. The same quantity of wood furnishes 50,000 cubic feet of gas, sufficient for the supply of 4000 lamps for five hours.”[20]
[20] See Repository of Arts, Vol. XI. No. 36, p. 341.
From what has been stated in the preceding pages it becomes obvious, that a substance yielding an artificial light may be obtained from common coal in immense quantities. The attempt to derive advantage from so valuable a discovery is surely no idle speculation. Let us therefore now consider to what objects of public and private utility this mode of procuring[100] light may be applied with effect. It is obvious that coal-gas may be preserved in a reservoir for any length of time and that it may be conveyed by means of tubes to any distance flowing equably and regularly like water. Those, indeed, who have not seen the contrivance will find it difficult to imagine with what ease it is managed. The gas may be distributed through an infinity of ramifications of tubes with the utmost facility. Near the termination of each of the tubes through which it flows, it is confined by a valve or stop-cock, upon turning which, when required to be lighted, it flows out in an equable stream and ascends by its specific levity. There is nothing to indicate its presence; no noise at the opening of the stop-cock or valve—no disturbance in the transparency of the atmosphere—it instantly bursts on the approach of a lighted taper, into a brilliant, noiseless, steady and beautiful flame. Its purity is attested by its not blacking or soiling in the least degree the metallic orifice from which it issues, nor even a sheet of white paper, or polished surface brought in contact with it. There is no escape of combustible matter unconsumed, which is so great a nuisance[101] in all our common lights. The products of the combustion are water and carbonic acid gas[21]. The accurate and elegant experiments of Dr. W. Henry have shewn in the most satisfactory manner, that considerably less carbonic acid is produced by the flame of coal-gas, than by that of oil, tallow, or wax[22], which[102] sufficiently refutes the absurd notions that have been circulated respecting the pernicious effects of gas-lights. But if the gas from Newcastle coal is badly prepared, or not deprived of the portion of sulphuretted hydrogen, which it usually contains, it then emits fiery sparks and produces a portion of sulphureous acid by virtue of the union of the oxygen of the air with the sulphur dissolved in the gas, the consequence of which is, a suffocating odour, which is particularly observable in the higher stratum of the air of apartments in which the gas is burnt. Such gas likewise tarnishes all metallic bodies—it discolours the paintings effected with metallic oxids, and always produces a suffocating odour very noxious to health. It is freed from the sulphuretted hydrogen and may be rendered fit for illumination by passing it repeatedly through very dilute solutions of sub-acetate of lead, green sulphate of iron, quicklime and water, or hyper-oxymuriate of lime.
[21] The water (which passes off in imperceptible vapour) is generated by part of the oxygen of the air uniting with part of the hydrogen, which forms the great bulk of the coal-gas: and the carbonic acid gas is produced by the union of another portion of the oxygen uniting with the smaller portion of carbon, which is the other component part of the coal-gas.
[22] 100 Cubic inches of carburetted hydrogen from coal, require for burning 220 cubic inches of oxygen and produce 100 cubic inches of carbonic acid—100 cubic inches of the same gas obtained from wax, require for burning 280 cubic inches of oxygen and produce 137 cubic inches of carbonic acid—100 cubic inches of the same gas procured from lamp-oil, require 190 cubic inches of oxygen for burning, and produce 124 cubic inches of carbonic acid.
The following lines relating to the salubrity of the gas-light illumination are copied from Mr. Lee’s evidence in the House of Commons, when examined on that subject.
Question—“Is the health of your manufacturers at all affected by the use of gas?—Answer—Not in the least, or I would not have adopted it. I believe I explained to the Committee, that I used the gas-lights in my own house first.”
Q. “You have not seen the smallest alteration in the health of your workmen?—A. Not in the least, for had I seen it, it would have been a fatal objection to it.”
Q. “And you say the same in regard to the use of the gas-lights in your own family?—A. Certainly I do.”
As to the brilliancy of the flame, an appeal may be made to every one who has witnessed the gas-light illumination, whether it be not superior to the best wax candle-light, or the light of Argand’s lamps.
It may be described as a rich compact flame, burning with a white and agreeable light. It is also perfectly steady, when the flame is limited to a moderate size: in large masses, it is subject to that undulation which is common to it with all flames of certain dimensions, and is caused by the agitation of the surrounding atmosphere. The gas flame is entirely free from smell. The coal-gas itself certainly has a disagreeable foetid odour before it is burnt, so has the vapour of wax, oil, and tallow, as it comes from a lamp or candle newly blown out. This concession proves nothing against the flame of gas which is perfectly inodorous, a white handkerchief, passed repeatedly through it and applied to the nose, excites no odour.
Another peculiar advantage of the gas flame is, that it may be applied in any direction we please, as there is nothing to spill and the gas is propelled by a certain force which is always the same, it will burn equally well in an almost horizontal as in an upright position; and we can thus obviate two great objections to all our artificial lights, that their least luminous end is directed downwards where the light is generally most wanted, and that a shade is cast below[104] by the stand or support of the combustible matter.
The size, shape and intensity of the gas-flame may be regulated by simply turning a stop-cock which supplies the gas to the burner. It may at command be made to burn with an intensity sufficient to illuminate every corner of a room, or so low and dim as barely to be perceived. It is unnecessary to point out how valuable such lights may be in nurseries, stables, warehouses, in the chambers of the sick, &c.
From the facility with which the gas-flame can be conveyed in any direction, from the diversified application, size and shape which the flame can be made to assume, there is no other kind of light so well calculated for being made the subject of splendid illuminations.
Where lustres are required in the middle of a room, the best mode of conducting the gas to the chandelier, is to pass the gas-pipe through the ceiling from the room above, immediately over the lustre. This can be easily done without injury to the apartment.
Where side-lights and chandeliers are required the tubes need never appear in sight, but may be concealed in the wall or floor of the[105] house. When transparencies are wanted as decorations for halls, lobbies, &c. more than light, recesses may be filled with different coloured media, or paintings, and any intensity of light may be thrown on the object.
If a number of minute holes are made in the end of a gas pipe, it forms as many jets de feu, which have a very brilliant appearance; these may sometimes be placed in the focus of a parabolic reflector. In cases where the light is required to be thrown to a distance, other burners are constructed upon the same principle as the Argand lamp, forming a cylinder of flame, and admitting a current of air both to the inside and outside.
On comparing the flame of a gas-light with the flame of a candle whatever its size may be, it appears just as yellow and dull as the flame of a common lamp appears when compared with that of a lamp of Argand. The beautiful whiteness of gas-light never fails to excite the surprize and admiration of those who behold it for the first time.
A large edifice or manufactory lighted by gas, contrasted with one of the same kind lighted by candles or lamps, resembles a street on the[106] night of a general illumination, compared with the glimmering light of its ordinary parish lamps.
The intensity of one of the parish gas-light lamps, now exhibited in the streets of this metropolis, will bear ample testimony of this assertion; the light of the parish gas-lamps, is to the intensity of the parish oil lamps as 1 to 12.
One of the most obvious applications of the gas-light illumination unquestionably consists in lighting streets, shops and houses; and let it be observed that as this is found safe and economical, it proves all that the most ardent friends of the gas-light system can desire. For in contending with the common mode of lighting the streets and shops, the new lights must beat out of the market the cheapest of all artificial lights; and as it has succeeded in doing this it shews in the most satisfactory point of view, the prodigious advantages of gas-lights when compared with the materials of tallow and oil.
The original expence of laying the pipes for conveying the gas, together with the cost of the machinery, is all that is required; the preparation of the gas being itself a lucrative process,[107] no doubt will pay all its expences besides the interest of capital, and leave a surplus of profit.
Indeed the application of the coal-gas, as a substitute for tallow and oil, to illuminate houses, shops, &c. is no longer problematical, a considerable extent of this capital, together with numerous shops and houses being already supplied with this species of light.[23]
[23] The Liberty of Norton Falgate, as far as Bishopgate-street, is lighted with gas-light, from the Chartered Company’s station at Norton Falgate; and gas-light pipes are laid from that station as far as the west end of Cheapside, and in all the streets north of that great thoroughfare.
In the West end of the Town, the main pipes for supplying the streets and houses with light from the Gas-Light Company, extend through the most eligible parts; from their Establishment in Peter-street, Westminster, along the line from Pall Mall to Temple-bar, compleatly surrounding the parish of St. Martin’s in the Field. Main pipes are also placed in the Hay-market, Coventry-street, Long-Acre, St. Martin’s-lane; and in the principal parts of the parishes of St. James and St. Ann.
In the East end of the metropolis, the gas-light mains extend from Cornhill to St. Paul’s, Wood-street, Fore-street, &c.—Consent has also been given to the incorporated Gas-Light Company for laying their pipes in the parish of St. Stephen’s in the Field; St. Paul Covent-garden; St. Mary-le-Strand; St. Clement Danes; St. George’s, Bloomsbury; St. Giles’s in the Fields; St. Andrew’s, Holborn, above the bars; part of the parish of St. Mary-la-bonne; besides several other districts, comprehending the whole of the city and suburbs of Westminster.
Enough therefore, has been done to prove the possibility of lighting houses, and streets, with[108] gas, which would have been regarded twenty years ago as an extravagant paradox.[24]
[24] I am informed by Mr. Clegg, the engineer of the Chartered Gas-Light Company, under whose direction the new system of lighting is carried on, that the total length of pipe laid down, as mains, in the streets of London amounts already to nearly 15 miles.
In the Eastern part of London, the same Company is engaged to lay their pipes in the principal parts of Whitechapel, Spitalfields, St. Luke’s, and the adjoining neighbourhood.
One part of the city of London, extending from Temple-bar to the West end of Cheapside; from Newgate-street to Holborn Bars, together with the intervening streets, is also provided with pipes laid down by another gas-light association, who have opened a new Establishment in Water-lane, Fleet-street, but are unconnected with the Chartered Company. A third company is projected in Southwark, and a fourth in the Eastern district of London, creating by a rivalry of interest, that laudable competition which always proves beneficial to the public at large, and which cannot fail to accelerate the progress of this new art of procuring light.
The Church of St. John the Evangelist in this metropolis has been illuminated with gas-lights for upwards of two years: the lights employed in this edifice is equal to 360 tallow candles eight to the pound. The avenues to the House of Lords and House of Commons, Westminster-hall, Westminster-bridge; the house and offices of the Speaker of the House of Commons, the Mansion-house, and many other places, deserve to be named, as having already adopted this species of illumination.
Another advantageous application of the gas-light must be the supplying of light-houses.
From the splendour and distinguishing forms which the gas-light flame is capable of assuming, no light is better calculated for signal-lights than this. By means of one single furnace as much gas might readily be procured as would furnish a flame of sufficient intensity, during the longest winter night, exceeding in brilliancy or intensity of light any light-house in Britain or elsewhere.
If every light-house round this island were possessed of a gas-light furnace, one-half part of the enormous expence which they at present require would furnish a much more brilliant light. The cheapness of this light and its efficacy for the purpose, would soon multiply the number of light-houses, and thus most essentially contribute to the security of navigation on our coast. The gas may be made to issue from tubes by long narrow slips, and a surface of flame produced of any given dimensions, and free from all smoke that would obscure the reflectors.
The ease with which the largest gas-light flame is instantly extinguished by shutting the[110] stop-cock, and the readiness with which a long line of gas catches fire by applying a lighted taper to one extremity, are properties that cannot fail to recommend it for the purposes of telegraphic communications by night. Another application of the gas unquestionably might be the lighting of barracks, arsenals, dock-yards, and other establishments where much light is wanted in a small place.
The annual expence of lighting the barracks of Great Britain is said to fall little short of 50,000l. a small part of which on the new plan, would supply them with a much purer and safer light.
The uses of the gas-lights already enumerated must of themselves, justify us in attaching great importance to the discovery, and if reduced to practice all over the kingdom, would employ a large capital in a way the most advantageous and productive. But the utility of this light will be almost indefinitely increased to the use of private families. That such an application is practicable, in all towns of Great Britain, is obvious, from what has been done already, and that it would be highly economical and ornamental, there can be little doubt.
By means of gas we may have a pure and agreeable light at command in every room of our house, just as we have the command of water, with this singular advantage, that these lights may burn for hours within an inch of the most combustible substance without danger, because they neither can burn down like a candle nor emit sparks. These properties make the gas-lights a most desirable light on board our ships of war, where severe regulations are necessary to prevent danger from fire, which after all are frequently evaded. The gas-light might be used in the store-rooms, and even in the powder magazine, and the captain would completely command the supply of light by the possession of the key which opens and shuts the stop-cock. A small apparatus which may be erected at a trifling expence would be sufficient for that purpose.
In shops, counting-houses, and public offices, the advantages are a white light, nearly equal to day-light, a warmth which almost supersedes the use of fires, a total absence of smoke, smell, and vapour, and great economy of labour.
The heat produced by gas-lights must be observed by every one who has had an opportunity[112] of attending to it in the most superficial manner, and the reason why gas-lights produce more heat than oil or candle-light will not appear strange to our chemical readers (and who is there now that does not know something of chemistry?) when it is considered that the gas-light flame condenses more air than the flame of oil and tallow, and consequently must produce more heat.
The flame of gas may be produced in so large a surface, as to be applied to heat the most spacious apartments as well as to light them.
If the gas is made to issue by a circular rim of about twelve inches diameter; it forms a sort of an Argand lamp on a great scale, and it is manifest that a circumference of three feet of flame will heat the air very rapidly, and with such uniformity that we need no longer be exposed to the partial heating occasioned by the strong draft of a large fire. A lamp of this description in the centre of a large room, with a very small fire to secure a gradual renewal of the air would enable us to enjoy the most healthful and agreeable temperature.
From trials made on this subject, I am enabled[113] to state, that three Argand’s lamps, consuming five cubic feet of gas per hour, are sufficient to keep a room 10 feet square at a temperature of 55° Fahr. when the air without doors has a temperature of freezing.[25]
[25] Mr. Dalton’s method of ascertaining the comparative quantity or effects of heat evolved during the combustion of different inflammable gases, and other substances capable of burning with flame, as stated in his System of Chemistry, vol. I. p. 76, deserves to be recommended to those who are more immediately interested in this subject. The process, which is simple, easy, and accurate, is as follows:
Take a bladder of any size, (let us suppose for the sake of illustration, the bladder to hold or to be equal in capacity to 30,000 grains of water,) and having furnished it with a stop-cock and a small jet pipe, fill it with the combustible gas the heating power of which is to be tried. Take also a tinned iron vessel with a concave bottom of the same capacity, pour into it as much water as will make the vessel and water together equal to the above stated bulk of water in the bladder, viz. 30,000 grains. This being done, set fire to the gas at the orifice of the pipe, and bring the point of the flame under the bottom of the tinned vessel, and suffer it to burn there, by squeezing the bladder till the whole of the gas is consumed. The increase of temperature of the water in the tinned vessel being carefully noticed before and after the experiment, gives very accurately the heating power of the given bulk of the inflammable gas.
It was thus proved that—
| Olefiant gas raises an equal volume of water | 14° |
| Carburetted hidrogen, or coal gas | 10 |
| Carbonic oxid | 4 |
| Hidrogen | 5 |
| Spermaceti oil 10 grains burnt in a lamp raised 30,000 grains of water | 5 |
| Tallow | 5 |
| Wax | 5,75 |
| Oil of turpentine | 3 |
| Spirit of wine | 2 |
In all processes of the arts where a moderate heat is wanted the gas-light flame will be found very advantageous—even on a large scale this flame may be used with profit. It possesses advantages which cannot be obtained from flaming fuel, where much nicety is required; because no fuel can be managed like the flame of coal-gas. For it is well known, that when too[114] little air be given to flaming fuel it produces no flame, but sooty vapour; and if too much air be admitted to make those vapours break out into flame, the heat is often too violent. It is a fact, that flame, when produced in great quantity, and made to burn violently, by mixing with a proper portion of fresh air, driving it on the subject, and throwing it into whirls and eddies, thereby mixing the air with every part of the hot vapour, produces a very intense heat.
The great power of a gas-flame does not appear when we try small quantities of it, and[115] allow it to burn quietly, because the air is not intimately brought into contact with it, but acts only on the outside; and the quantity of burning matter in the surface of a small flame is too minute to produce much effect.
But when the flame is produced in large quantity and is freely brought forward into contact and agitated with air, its power to heat bodies is immensely increased. It is therefore peculiarly proper for heating large quantities of matter to a violent degree, especially if the contact of solid fuel with such matter is inconvenient.
As the gas-flame may be made to assume any shape and intensity, and as there is nothing to spill, it may be exhibited under such variety of forms and designs, as cannot fail to give rise to the most tasteful ornamental illumination.
Plates III. IV. and V. exhibit such designs of different kinds of gas-lamps, chandeliers, lustres, candelabras, &c. as are already in use in this Metropolis.
Plate III. fig. 1, represents a Rod Lamp. The gas passes through the rod a, to the Argand burner, which is surrounded by a cylindrical chimney, c, swelling out at the lower[116] extremity. The construction of the Argand burner we have mentioned already, p. 78.
In all the gas-light burners, constructed on Argand’s plan, care should be taken that the flame be in contact with the air on all sides, and that the current of air be directed towards the upper extremity of the flame. This may be effected by causing a current of air to rise up perpendicular from the bottom of the chimney glass, and to pass out again through the contracted part, or upper extremity of the chimney; but no other current of air should ever be permitted to come near the gas-flame, or enter the glass chimney which covers or defends the light; for if more air be permitted to mix with the flame than is sufficient for the compleat combustion of the coal-gas, it necessarily diminishes the heat, and consequently reduces the quantity of light.
Fig. 2. A Rod Gas Lamp, with branches. The gas passes through the hollow rod, a, and part of the hollow branch, b, to the burner of the lamp. The cylindrical shaped glass, c, exhibited in this figure, is not so well adapted for the compleat combustion of coal-gas, as the belly-shaped chimney, c, represented in[117] fig. 1, 3, 5, 6, because the ascending current of fresh air is not turned out of its perpendicular course, and thrown immediately in a concentrated state, into the upper part of the flame where the combustion of the gas is less perfect. The exterior current of air which enters at the bottom into the lamp, rises merely with a velocity proportioned to the length of the cylinder, and to the rarefaction of the air in the same, but without being propelled to the apex of the flame, as it should do, and is made to do, in the bellied glass adapted to the lamp, fig. 1.
Fig. 3. A Bracket Lamp. a, the tube which conveys the gas to the burner; b, the stop-cock of the tube.
Fig. 4. A Pendent Rod Lamp; in which the gas is supposed to come from a pipe above, through the ceiling, into the pipe, a, to supply the burners. The tulip-shaped chimney, b, of this lamp, is likewise ill adapted for gas-light burners.
Fig. 5. A pendent double-bracket Lamp. The gas passing through the perpendicular tube, a, into the brackets, b b; c shows the Argand burner.
Fig. 6. A swing Bracket Lamp. a, the gas-pipe with its stop-cock; b, a brass ball, communicating with the pipe, a; c, the conducting tube, ground air-tight into the ball, b, and communicating with the burner of the lamp, so as to allow it to have an horizontal motion.
Fig. 7. Shews the construction of the ball b, and pipe, c, of the lamp, fig. 6.
Fig. 8. A Swing Cockspur Lamp, constructed upon the same plan as fig. 6. These two lamps are very convenient for desks in counting-houses, &c.
Fig. 9. A stop-cock with ball and socket, which, when adapted to a gas-light pipe, allows it to have an universal motion, so that the light may be turned in any direction.
Fig. 10. Section of the stop-cock, with ball and socket.
Fig. 11. Shows the ball and socket, fig. 9, in perspective.
Plate IV,[26] fig. 1. A Candelabrum; the gas[119] pipe ascending from the floor of the apartment, through the column a, and terminating in the burner of the lamp.
[26] The gas-lamps exhibited in this plate, are employed in the library, counting-house, warehouse, and offices of Mr. Ackerman, and, by whose permission, they are copied on this occasion.
Fig. 2. A fancy pendent Cockspur Lamp. The gas being transmitted to the burners, c c, by means of the pipe, a.
Fig. 3. A Pedestal Argand Lamp. a, the pipe and stop-cock, which transmits to, and shuts off the gas from the burner of the lamp.
Fig. 4. A Pedestal Cockspur Lamp. a, the stop-cock and gas-pipe.
Fig. 5. A fancy bracket Cockspur Lamp, intended merely to show that the coal-gas, as it passes to the burner, is perfectly devoid of colour, and invisible. a is a glass vessel furnished at its orifice with a brass cap, c, and perforated ball, out of which the gas-flame proceeds. b, the pipe which conveys the gas into the glass vessel, a.
Fig. 6. A Bracket Argand Lamp. a and b, the gas pipe communicating with the burner.
Fig. 7 and 8. A Horizontal Bracket Lamp. a, the gas pipe, supposed to be concealed in the ceiling. b, the communicating pipe, which, together with c, branches out at right angles at d d. e e, are the burners of the lamp.
Plate V. fig. 1. A Candelabrum, into which the gas-pipe ascends from the floor of the apartment, the lateral branches communicating with the central tube.
Fig. 2. An Arabesque Chandelier. The gas enters from the ceiling of the room into the rope-shaped pipe, a, from which it proceeds through one of the arched ribs, b b, into the horizontal hoop, or pipe, c.
Fig. 3. A Roman Chandelier. The gas enters through the inflexible hollow chain, a, into the central tube, b, from whence the burners are supplied by the lateral branches, c c.
Fig. 4. A Gothic Chandelier. The gas is transmitted to the burners through the rope, a, which includes a tube, and the communication with the burners is established through the lateral branches.
Fig. 5. A Pedestal Figure Lamp. The gas is here made to pass by means of a pipe through the body of the figure into the lattice-work plateau, constructed of hollow and perforated brass tubes.
Fig. 6. A Pedestal Vase Lamp. The gas-tube enters through one of the claw-feet of the altar-shaped pedestal, into the glass vase, a, at[121] the bottom of which it joins the tubes communicating with the metallic corn-ears, b, at the upper extremities of which it forms jets de feu.
Fig. 7. A Girandole. The gas enters through the bracket, a, and is conveyed to the burners by the descending tubes, b b.
Fig. 8. A Candelabrum, having a central pipe, through which the gas is conducted to the burner at the top.
Having thus far considered the nature of coal-gas as a substitute for the lights now in use, it will be necessary to attend more particularly to some other products which are obtained during the production of this species of light: namely, coke, tar, ammoniacal liquor, &c.
Coke.—The substance called coke, which constitutes the skeleton of the coal, or its carbonaceous base, is left behind in the retort, after[122] all the evaporable products have been expelled from the coal by heat.—See page 85.
It is sufficiently known, that coke is a more valuable fuel than the coal from which it is obtained.
Hence, immense quantities are prepared in the large way, but the gazeous and other substances are lost in the process employed for carbonizing the coal.[27] In the manufacture[123] of coal-gas, the coke comes from the retort, enlarged in size, and greatly diminished in weight, when compared with the original coal. In whatever state the coal may be when introduced into the retort, the coke is uniformly taken out in large masses, so that the refuse coal, or dust, and sweepings of the pit, which are now thrown away, may be employed and converted into an excellent fuel. Coke is decidedly superior to coal for all domestic, and more especially for culinary purposes; the heat which it throws out being more uniform, more intense, and more durable. No flame, indeed, accompanies it, and it seldom needs the application of the poker,—that specific for the ennui of Englishmen; but these deficiences are more than balanced by the valuable property of emitting no sparks, of giving more heat, and burning free from dust and smoke.
[27] The preparation of coke is as follows:—A quantity of large coal is placed on the ground in a round heap, of from 12 to 15 feet in diameter, and about two feet in height; as many as possible of the large pieces are placed on their ends, to form passages for the air; above them are thrown the smaller pieces and coal dust, and in the midst of this circular heap, is left, a vacancy of a foot wide where a few faggots are deposited to kindle it. Four or five apertures of this kind are formed round the ring, particularly on the side exposed to the wind; there is, however, seldom occasion to light it with wood, for other masses being generally on fire, the workmen most frequently use a few shovels of coal already burning, which acts more rapidly than wood, and soon kindles the surrounding pile; as the fire spreads, the mass increases in bulk, puffs up, becomes spongy and light, cakes into one body, and at length loses its volatile parts, and emits no more smoke. It then acquires an uniform red colour, inclining a little to white, in which state it begins to break into gaps and chinks, and assumes the appearance of the under part of a mushroom; at this moment the heap must be quickly covered with ashes, of which there is always a sufficient provision around the numerous fires, where the coke is prepared.
That coke must give out more heat during its combustion than coal, will at once become obvious, when we consider that the quantity of matter which, in the combustion of coal is changed from a solid to a state of elastic fluidity, must necessarily carry off a portion[124] of caloric, which then becomes converted in a latent state without producing heat, whilst the glow of the coke radiates caloric with an intensity unimpaired by any demand of this kind.
It is thus that coke, though somewhat more difficult of ignition than common coal, always gives out a more steady, a more lasting, and a more intense heat.
The only inconveniences that attend the use of coke is, that, as it consumes, it leaves much more ashes than common coal, charcoal, or wood; and these much heavier too, which are, therefore, liable to collect in such quantity as to obstruct the free passage of air through the fire; and further, that when the heat is very intense, these ashes are disposed to melt or vitrify into a tenacious drossy substance, which clogs the grate, the sides of the furnace and the vessels. This last inconvenience is only troublesome, however, when the heat required is very great. In ordinary heats, such as are produced by kitchen or parlour grates, the ashes do not melt, and though they are more copious and heavy than those of charcoal or wood, they do not choke up[125] the fire, unless the bars of the grate be too close together.
The relative effects of heat produced by coke and coal are as follows:—
Six hundred pounds of pit-coal are capable of evaporating 10 cubic feet of water in 20 hours, and 430lb. of coke are capable of evaporating 17 cubic feet of water in 12 hours and a half.[28]
[28] In order to learn the relative effect of different kinds of fuel, with regard to their capability of producing heat, chemistry teaches that equal quantities of fuel alike expended, will raise the temperature of a given quantity of water through the same number of degrees; whence, by knowing the original quantity and temperature of water, together with the quantity of fuel expended to raise the water to the boiling point, the result sought may be expressed by stating the quantity of water at 30 degrees, which would have been raised 180 degrees by one pound of the fuel employed; or in the form of a rule,
Multiply the quantity of water by the number expressing the degrees actually raised; multiply the number of pounds of fuel expended by 180 degrees. Divide the first product by the latter, and the quotient will express the water which would have been raised 180 degrees by one pound of the fuel. Or equal quantities of water may be compleatly evaporated under equal surfaces and circumstances, with the different kinds of fuel, the nature of which is to be examined; the quantities of fuel expended for that purpose give the relative effect of the different kinds of fuel, with regard to their power of producing heat.
The Earl of Dundonald has shown that, in the application for burning lime, a quantity of coke uniformly burns a given portion of lime-stone in one-third part of the time that the quantity of coal from which the coke had been made could do.
This effect is to be accounted for from having previously freed the coal, or rather its coke, from the moisture and the tar, which it sends out during combustion, and which condenses on the middle and upper strata of stratified limestone and coal in the lime kiln, and impedes the whole mass of materials from coming into a rapid and compleat ignition; because the greater the quantity of materials, and the sooner the whole is ignited, the better and more economically the lime is burned, both as to coals and time; the saving of which last is a material object, especially at lime-kilns where there is in the summer time a great demand for lime, the coke occasioning the kilns to hold a third more lime at the same time.
In the art of making bricks, in the smelting of metallic ores, and the drying of malt, the advantages of coke over coal, are sufficiently known.
The following account given by Mr. Davis,[29] shows that the advantages that may be derived in the processes of burning lime, plaster of paris, and bricks, by means of coke, are greater than at first sight might be imagined.
[29] Philosophical Magazine, Vol. 33, p. 435.
“The coke obtained in the gas process is so valuable, that it appears inexplicable that men should not avail themselves of this mode of procuring light, to the almost total exclusion of all other methods now in use. As a landholder, placed among an industrious but wholly illiterate society of men, I have had the more opportunity of trying this species of fuel or coke, which I could not otherwise procure in this sequestered spot, at a tolerably cheap rate, for purposes to which it has not, as far as I know, been hitherto employed. I must tell you that I am my own lime-burner, plaster of paris baker, and brick-maker; and that in these processes of rural economy I have derived the greatest benefits from this species of fuel, which I now prepare at a cheap rate, although I waste almost the whole of the light of the coal gas intentionally. The coal which I employed formerly for the burning[128] of limestone into lime, is a very inferior kind of small coal, called here Welsh culm. The kiln for burning the limestone into lime is a cup-shaped concavity, surrounded with solid brick-work, open at the top, and terminating below by an iron grate. It has a stone door that may be opened and closed for charging and emptying the furnace when required. This furnace I formerly charged with alternate strata or layers of small coal and limestone, the latter being broken previously into pieces not larger than a man’s fist, until the kiln was completely filled. The stone is thus slowly decomposed; the upper part of the charge descends, and when it has arrived at the bottom of the furnace new strata are super-imposed, so as to keep the furnace continually full during a period of 50 hours. The quantity of lime I procured with small coal formerly amounted to 85 bushels. The strata of coal necessary for the production of this quantity of lime require to be four inches thick, and the time necessary for calcination was, as stated already, 50 hours.
“On applying coke instead of coal, the produce of lime may be increased to nearly 30[129] per cent. from the same furnace, and the time required to effect the calcination of this quantity of lime-stone is reduced to 39 hours: it also requires less attendance and less labour, and the whole saving, thus accomplished, amounts to more than 50 per cent. on the lime-kiln.
“I have lately also employed coke for the burning of bricks. My bricks are burnt in clamps, made of bricks themselves. The place for the fuel, or fire-place, is perpendicular, about three feet high. The flues are formed by gathering or arching the bricks over, so as to leave a space between each of a brick’s breadth; and as the whole of the coal, if this fuel be employed, must, on account of the construction of the pile, be put in at once, the charge of the bricks is not, and never can be, burnt properly throughout; and the interference of the legislature, with regard to the measurement of the clamp, is a sufficient inducement for the manufacturer to allow no more space for coal than he can possibly spare.
“If coke be applied instead of coal, the arches, or empty spaces in the clamp or pile,[130] as well as the strata of the fuel, may be considerably smaller: the heat produced in this case is more uniform and more intense, and a saving of 30 per cent. at least is gained.
“In the baking my own plaster-stone I also employ coke. The calcination of the stone for manure I perform in a common reverberatory furnace, and the men who conduct the process (who are otherwise averse to every thing new) are much pleased with the steadiness of the fire, and little attendance which the process requires, when coke is used instead of coal.
“These are the few facts I wish to state, with regard to the useful application of this species of fuel, which, no doubt, hereafter will become an object of economy of incalculable advantage to individuals, if its nature be better understood than it is at present.”
The quantity of coke obtainable from a given quantity of coal varies according to the nature of the coal employed. One chaldron of Newcastle coal produced, upon an average, in the gas-light manufacture, from one chaldron and a quarter to one chaldron and a half of well formed coke. If the carbonization of the coal has been carried to its utmost point,[131] the coke produced, has a brilliant silvery lustre. Such coke is excellent for metallurgical operations, because it stands the powerful blast of the bellows, but for culinary and other purposes of domestic economy, the carbonization should not be carried so far, because, the coke then produced, kindles more readily and makes a more cheerful fire.
Coal-tar, Oil, and Pitch.—Another, valuable product obtainable from pit-coal, is coal-tar.[30] This substance is deposited, in the purification of the coal-gas, in a separate vessel destined to receive it.
[30] In the year 1665, Becher, a German chemist, brought to England his discovery for extracting tar from coal, this distillation he performed in close vessels. It is not mentioned in the records of the time, whether Becher obtained, or rather collected, any other articles than the tar.
The coal-tar is so called from its resembling common tar in its appearance, and most of its qualities.
Several works have been, at different times, erected both in England and on the continent, to procure from coal a substitute for tar; but they turned out unprofitable speculations. In 1781, the Earl of Dundonald invented a mode of distilling coal in the large way, which enabled[132] him not only to form coke, but, at the same time, to save and collect the tar. Even this process however, for which a patent was taken out, has gained very little ground. Its object was still too limited; for though some of the ingredients of coal were procured, they were procured at an expense that nearly balanced the profits; and no attention whatever was paid to the coal gas, which constitutes the most important part of coal.
Coal-tar may be used with advantage for painting and securing wood that is exposed to the action of air or water. The wood being warmed, the tar is applied cold, and penetrating into the pores, gives the timber an uncommon degree of hardness and durability.
One chaldron of Newcastle coal produces in the gas-light manufacture from 150 to 180lb of tar, according to the circumstances under which it is produced. See page 94.
The tar obtained from Newcastle coal-tar is specifically heavier than that produced from cannel-coal; hence it sinks in water, whereas the latter swims on the surface of that fluid.
To render the tar fit for use, it requires to be evaporated to give it a sufficient consistence.[133] If this process be performed in close vessels, a portion of an essential oil is obtained, which is known to colourmen by the name of oil of tar. To obtain this oil, a common still is filled with the coal-tar, and, being properly luted, the fire is kindled and kept up very moderate, for the tar is very apt to boil up in the early part of the process. The first product that distils over is principally a brown ammoniacal fluid, mixed however with a good deal of oil. As the process advances, and the heat is increased, the quantity of ammoniacal liquor lessens, and that of oil increases, and towards the end of the distillation the product is chiefly oil.
The oil and ammoniacal water which distil over do not mix, so that they may be easily separated by decantation. The oil is a yellowish inferior kind of oil of turpentine, which is very useful in painting ships, for making varnishes, and other coarse out-door work.
Two hundred pounds of tar produce, upon an average, fifty-three pounds of essential oil.
If the coal-tar is wanted to be converted into pitch, without obtaining the oil which it is capable of furnishing, the evaporation of it[134] may be performed in a common boiler; but as it is extremely liable to boil over, the greatest precaution is necessary in conducting the evaporation. A boiler constructed on the following plan is very convenient for the conversion of coal-tar into pitch. The contrivance consists in adding a spout, or rim, to the common boiler, into which the tar spreads itself as it rises, and by this means becomes cooled, and the boiling over is checked.
Kettle for boiling Tar.
1000lb. of coal-tar produce, upon an average, from 460 to 480lb. of pitch. A subsequent fusion, with a gentle heat, converts the coal-pitch into a substance possessing all the characters of asphaltum.
Ammoniacal Fluid.—The properties of the ammoniacal liquor, which accompanies the tar,[135] and which is deposited in the tar-cistern, has not yet been fully investigated. It is employed already in the manufacture of muriate of ammonia (sal ammoniac). One chaldron of coal affords from 220 to 240lb. of this ammoniacal fluid, which is composed chiefly of sulphate, and carbonate of ammonia.—Such are the products obtainable from coal.
However certain the practicability of extending the new lights to the dwelling houses of every town and village is, it cannot be expected that such an event should take place speedily and generally. To eradicate prejudice, and to alter established habits, is a work which nothing but time can effect; because prejudice is the effect of habit, and can seldom be eradicated from the minds of such individuals as consider the ready occurrence of a proposition as a test of its truth. To establish a new philosophical theory has, in every instance, required time sufficient to educate an entire generation of men. The rejection of the Aristotelian philosophy—the adoption of experimental research—the substitution of the doctrine of gravitation instead of that of vortices, and the rejection of phlogiston by modern chemists, are[136] sufficiently illustrative of this assertion. New arts, and new practices, are still more difficult to be introduced. The new art of bleaching need merely be mentioned to prove this assertion. The new grammar—the new rudiments of science—the new stile—or the new instrument, however superior to the old in simplicity, facility, and truth, must be less valuable to the ordinary teacher or artisan, whose memory is familiarized with the precepts of the latter, and whose only ambition is to earn his subsistence with the least possible exertion.
The slowness with which improvements of every kind, make their way into common use, and especially such discoveries as are most calculated to be of an extended or general utility is very remarkable, and forms a striking contrast to the extreme avidity with which those unmeaning changes are adopted, which folly and caprice are continually sending forth into the world under the auspices of fashion.
On the first view of the subject it appears very extraordinary, that any person should neglect, or refuse to avail himself of a proposed invention, or improvement, which is evidently calculated to economise his labour,[137] and to encrease his comforts; but when we reflect on the power of habit, and consider how difficult it is for a person even to perceive the disadvantages or imperfections of former modes to which he has been accustomed from his early youth, our surprize will be diminished, or vanish altogether.
Many other circumstances, besides prejudice, are unfavourable to the introduction of new and useful discoveries. Among these jealousy, malice, envy, and revenge, have too often their share in obstructing the progress of real improvement, and in preventing the adoption of plans evidently calculated to promote the public good.
A plan like the present, which proposes not only to trench upon domestic habits, but to give an entire new direction to a portion of the skill and capital of the country, must necessarily encounter the most strenuous opposition. It is thus that some individuals have mustered all their strength against the introduction of this new art. An endeavour has been made to move the public opinion by dismal forebodings of the Greenland trade, and the subsequent loss of a nursery of British seamen. This objection[138] is nothing more than the common clamour that is always set up against every new means of abridging labour, to which had the public listened, an interdict would have been laid upon the spinning and threshing machines, the steam engine, and a thousand other improvements in machinery.
Indeed such clamour scarcely ever fails to be made when the extension of machinery and the abridgement of labour or the application of inanimate powers are considered. On such occasions, it is stated by certain humane but mistaken objectors, that the scheme of mechanical and chemical improvement is pointed against the human species—that it tends to drive them out of the system of beneficial employment—that the introduction of machinery is injurious to the labouring class of society, by abridging their work. Two creatures offer themselves for employment and support—a man and a horse. I must invariably prefer the latter, and leave the former to starve. Two other beings—a horse and a steam-engine, are candidates for my favour. My preference to the latter tends to exterminate the species of the former. In both cases it is stated, that the number of intelligent[139] creatures capable of the enjoyment of happiness must be diminished for want of support; and that, on the whole, the sum of the proposed improvement is not only a less proportion of good to society, but a positive accession of misery to the unemployed poor.
On this wide and extended argument, which can in fact be maintained against all improvements whatever in no other way than by insisting that the savage state of man, with all its wants, its ignorance, its ferocity, and its privations, is preferable to the social intercourse of effort and division of labour we are habituated to prefer, it may be sufficient to observe that it includes matter not only for reasoning and induction, but also for experiment. By reference to the matter of fact, though it must be allowed that new improvements, which change the habits of the poor, must at first expose them to a temporary inconvenience and distress, against which, in fairness, it is the duty of society to defend them; yet the invariable result of such improvements is always to better the condition of mankind. A temporary inconvenience to individuals must often be incurred for the sake of general national benefit.
It is to manufactories carried on by machinery and to the abridgment of labour, that this country is indebted for her riches, her independence and pre-eminent station among the nations of the world.
But let us return to the subject.—The progress of the new mode of lighting with coal-gas can never wholly supersede the use of candles and moveable lights. The objection with regard to the Greenland trade is equally futile. This traffic, might with more propriety be called a drain, than a nursery, of the naval force. The nature of the Greenland service requires that the crew should consist chiefly of able-bodied sailors; and being protected men, not subject to the impress law, they are thus rendered useless for national defence. The nursery of British seamen is the coasting trade; and if the gas-light illumination be put in practice to a large extent, it will increase that trade as much as it will diminish the Greenland fishery.
Even on the extreme supposition that it would annihilate the Greenland fisheries altogether, we should have no reason to regret the event. The soundest principles of political[141] economy must condemn the practice of fitting out vessels to navigate the polar seas for oil, if we can extract a superior material for procuring light at a cheaper rate from the produce of our own soil.
Indeed the fisheries will find ample encouragement, and the consequence of lighting our streets with gas can prove injurious only to our continental friends, one of whose staple commodities, tallow, we shall then have less occasion to purchase.
There will be less waste indeed, but a greater consumption of coal. The lower classes of the community are at present very scantily supplied with firing; and nothing but a reduction of price is necessary to increase to a very large amount the whole average quantity of fuel consumed in the country. The lightness of the coke produced in the gas-light manufacture diminishing the expence of land carriage, will facilitate its general diffusion—the comforts of the poor will be materially augmented, and a number of useful operations in agriculture and the arts be carried on, which are now checked and impeded by the price of fuel.
If any additional want were wanted for the[142] coke it will readily be found in the continental market; coke being much better suited than coal to the habits of most European nations.
The gas-light illumination cannot tend to diminish the coal-trade; on the contrary it will prove beneficial to it; it will contribute to lower the price of the superior kinds of coal, and keep a level which cannot be shaken under any circumstances; it will contribute to prevent combinations which do certainly operate to the prejudice of the public, and do sometimes put this great town at the mercy of particular proprietors in the north, who deal out coal in the way they please. The competition thus produced, it is impossible not to consider as an advantage, which would prevent in future such combinations, and put those in London out of the reach of them.
It is worthy observation, that the annual importation of coal into this Metropolis, is above one million and eighty-eight thousand chaldrons.[31]
[31] To give an idea how long there is a probability of Great Britain being applied with coal from the rivers Tyne and Wear only, it must be observed,
1st. That the Seams of coal which are now worked at Newcastle and Sunderland, are equal to a seam or bed of 15 miles by 20 miles.
2dly. That this seam, on an average, is at least four feet and a half thick.
3dly, That 1-6th part of the above extent is sufficient for pillars to support the roofs of the mines, &c.
And, 4thly, It appears, by experiments, that a cubic yard of coal weighs 1 ton, or 20 cwt.
| London Chaldrons | |
| The total consumption of coal from the rivers Tyne and Wear known from the register to be | 2,300,000 |
| The number of tons in the above quantity taking the London chaldron at 27 cwt. is | 3,100,000 |
| Now a ton weight of coal is estimated to occupy in the earth the space of one cubic yard. | |
| The number of cubic yards in the square mile is | 3,097,600 |
| The beds or seams of coal are, on an average, 4 feet and a half in thickness, which increases the above number of cubic yards in the square mile by half the number of square yards to | 1,548,800 |
| And hence the square mile of the beds or seams of coal we are describing contains, of cubic yards and tons of coal | 4,645,000 |
| A deduction of 1-6th for pillars to support the mine, &c. | 800,000 |
| The number of tons per square mile | 5,445,000 |
We have already mentioned the length and breadth of the seams of coal to be equal to 20 miles by 15, making an area of 300 square miles, and consequently a source of consumption for 375 years.
It may be objected to the universality of our conclusion, that the price of coals, differing very much in different places, will occasion a variation in the expence of the new mode of[144] illumination. But there are two reasons why this should have less place, because we find, in Mr. Murdoch’s statement, page 69, that of 600l. the estimated yearly expence of lighting the cotton mill, 550l. consist of interest of capital, and tear and wear of apparatus, leaving the cost of coal only 50l. a sum so trifling, when we reflect that it replaces 2000l. worth of candles, that the price of coal, even where it is highest, can but slightly affect the general profits.[32]
2dly, The coal, by yielding the gas and other products,—namely, tar, pitch, ammoniacal liquor, &c. of which we have treated already, is converted into a substance, increased in bulk, and in the power of producing heat, namely, coke; and as a manufactory generally requires heating as well as lighting, there will be a gain both ways. The manufacturer, by distilling his coal, instead of burning it as it comes from the pit, will save his candles and improve his fuel. One effort at the outset, in erecting a proper apparatus, will reduce his annual disbursement, for these two articles of prime necessity,[145] much in the same manner, (though in a far greater degree) as the farmer gains by building a thrashing machine and laying aside the use of the flail.
The principal expence in the pursuit of this branch of civil and domestic economy is therefore the dead capital employed in erecting the machinery destined for preparing and conveying the gas; the floating or live capital is comparatively small. At the same time, were we to offer an advice to the public on this subject, it would be, that no private individual resident in London should attempt to light his premises for the sake of economy with coal-gas by means of his own apparatus, whose annual expence for light does not exceed 60l. because the expence of erecting and attending a small apparatus is almost as great as one constructed on a larger scale would be. For if the quantity of gas wanted is not sufficient to keep the retorts continually in a red-hot or working state, the cost of the gas will be considerably enhanced; because either the empty retorts must be continued red-hot, or the fire must be suffered to go out; and the retorts, when cold, cannot be[146] brought to a working state, that is to say, be made red hot again, but at a considerable expence of fuel, which must be wasted to no purpose. Whereas, if the retorts are constantly kept red hot and in action, one half of the coal necessary to produce a given quantity of gas will then be saved. But when a street, or a small neighbourhood is wanted to be lighted, and the retorts can always be kept in a working state, that is to say, red hot, the operation may be commenced with safety; because the sum required for erecting the apparatus, and the labour attending it, together with the interest of money sunk, will then soon be liquidated by the light which it will afford.
Individuals, therefore, may engage in the distillation of coal, and trade with advantage in the articles produced by that process, and the lighting of cities may be accomplished without the aid of incorporated bodies; and parishes may be lighted by almost as many individuals as there are streets in a parish.
From experiments, made by Mr. Clegg, on the effects produced by a number of gas-lights, of a certain intensity, there is reason to believe[147] that the streets of small towns might be illuminated at a cheaper rate, by means of a tower, or pagoda, furnished with gas-lamps, than can be done in the ordinary way by street lamps: the gas being conducted to the top of the building from the apparatus below, and the light directed down again, upon the objects to be illuminated, by means of reflectors placed at a certain angle. By this contrivance, all the main pipes which convey the gas through the streets, as well as those collateral ones that branch out from them to the street lamps, would be saved, and thus compensate for the expense of the tower.
The most beneficial application of gas-lights unquestionably is in all those situations where a great quantity of light is wanted in a small place: and where light is required to be most diffused, the advantages of this mode of illumination are the least.—Hence, as already stated, the lighting of the parish, or street-lamps only, without lighting shops or houses, can never be accomplished with economy.
We have noticed before the reason why the price of coals can have little effect upon the gas-light; because the very refuse, or small[148] coals, called slack, which pass through the screen at the pit’s mouth, and which cannot be brought into the market—nay, even the sweepings of the pit, which are thrown away, may be employed for the production of coal-gas. It makes no difference in what form the coal is used, and this circumstance may contribute to enable the coal-merchant to furnish coals in larger masses, and as they come from the mine, instead of increasing the bulk by breaking them into a smaller size,[33] which is a practice commonly[149] adhered to. This unquestionably reduces the value of coals; because the quantity of radiant heat generated in the combustion of a given quantity of any kind of fuel depends much upon the management of the fire, or upon the manner in which the fuel is consumed. When the fire burns bright, much radiant heat will be sent off from it; but when[150] it is smothered up, very little will be generated: most of the heat produced will then be expended in giving elasticity to a thick dense vapour, or smoke, which is seen rising from the fire; and the combustion being very incomplete, the carburetted hidrogen gas of the coal being driven up the chimney without being inflamed, the fuel is wasted to little purpose.
[33] It is not generally apprehended, how very wasteful the use of small coals is in the ordinary open fire-grates. Necessity makes us use the poker very much, particularly, when the coals are small; and habit prevails even when they are large. By the constant stirring of the fire almost the whole of the small coal passes through the bars; and consequently a great deal goes to the dust-hole without being burnt at all. To prove this, we need only take a shovel full of ashes and put them into a pail, and then pouring water over them, which being gently run off, will carry away nearly all the light and burnt parts: and leave an astonishing quantity of bright unburnt coal, which has escaped from the fire-place, in consequence of being small.
When the grate of the fire-place is large, and the small coals are thrown behind; or when we can have patience enough to bear the cold for an hour or two, or contrive to have the fire lighted a long time before we want it, the small coal may be of some use, but the fire made with it is never strong, nor so bright; and does not burn so long as a fire made with large or round coals: it often requires the help of the poker, and produces a great quantity of breeze.
The loss in the use of small coals is more considerable to the poor, who cannot keep large fires. When they want their breakfast or dinner, the time they can spare is limited; and to have their water sooner boiling, or their meals quicker ready; they must make use of the poker, and lose a great deal of coal. This fact is so evident, that any body who wishes to make the experiment before recommended, will find that much more bright coal goes to the dust-hole of the poor man, than to the dust-hole of a rich family, where, the fire-place being large, the small coal has more chance of burning.
The loss is still greater to the poor, in consequence of the inferior sorts of coal which are sold to them. If it is the light sort, it burns too quick, and they consume double the quantity; if the strong sort, it burns too slow, and is nearly as wasteful; for a great quantity of it then goes to the dust-hole without having been lighted at all.
An incorrect opinion is often entertained, that the real quantity of coal contained in a sack is lessened by separating or screening the small from the round coals; but we must recollect, that any compact body occupies less space than is required to contain the same matter, reduced to smaller irregular pieces, or to powder.—Now the screening only takes away the finest dusty part of the coals, and admits more small pieces of round coals to be filled into the sack.
Nothing can be more perfectly devoid of common sense, and wasteful and slovenly at the same time, than the manner in which chimney fires, where coals are burnt, are commonly managed by servants. They throw on a load of (perhaps all small) coals at once, through which the flame is hours in making its way; and frequently it is not without much care and trouble that the fire is prevented from going quite out. During this time no heat is communicated to the room; and, what is still worse, the throat of the chimney being occupied merely by a heavy dense vapour, not possessed of any heating power, and, consequently, not having much elasticity, the warm air of the room finds less difficulty in forcing its way up the chimney and escaping,[151] than when the fire burns bright, and the coal-gas is ignited. And it happens not unfrequently, especially in chimnies and fire-places ill-constructed, that this current of warm air from the room which presses into the chimney, crossing upon the current of heavy smoke and aqueous vapour which escapes slowly from the fire, obstructs it in its ascent, and beats it back into the room. Hence it is that chimnies so often smoke when too large a quantity of fresh coals is put upon the fire. So many coals should never be put on the fire at once as to prevent the free passage of the flame between them, or to prevent them becoming quickly heated, so as to give out the carburetted hidrogen gas which they are capable of furnishing, and to cause it to be inflamed, In short, a fire should never be smothered: and when attention is paid to the quantity of coals put on, there is little use for the poker; and this circumstance will contribute much to cleanliness, and the preservation of furniture.
The author of a paper in the Plain Dealer asserts, that, of the various perversions of abilities, there is none that makes a human being more ridiculous, than that of attempting[152] to stir a fire without judgment; to prevent which he lays down the following rules:—1. Stirring of a fire is of use, because it makes a hollow where, the air being rarefied by the adjacent heat, the surrounding air rushes into this hollow, and gives life and support to the fire, and carries the flame with it. 2. Never stir a fire when fresh coals are laid on, particularly when they are very small, because they immediately fall into the hollow place, and therefore ruin the fire. 3. Always keep the bottom bars clear. 4. Never begin to stir the fire at the top, unless when the bottom is quite clear, and the top only wants breaking.
There is one subject more on which it is necessary to speak.—In the present instance, the public has been alarmed by representations that the general adoption of gas-lights would expose us to innumerable accidents, from the inflammable nature of the gas, and the explosion of the apparatus in which it is prepared, or the bursting of the pipes by which it is conveyed. But there is no ground for such fears.
Those who are familiar with the subject will readily allow, that there is no more risk[153] in the action of a gas-light machinery, properly constructed, than there is in the action of a steam-engine, built on just principles.
The manufacture of the coal-gas requires nothing more than what the most ignorant person, with a common degree of care and attention, is competent to perform. The heating of the gas-furnace, the charging of the retorts with coal, the closing them up air-tight, the keeping them red-hot, and discharging them again, are the only operations required in this art; and these, surely, demand no more skill than a few practical lessons can teach to the meanest capacity. The workman is not called upon to exercise his own judgment, because, when the fire is properly managed, the evolution of the gas goes on spontaneously, and without further care, till all the gas is extricated from the coal.
No part of the machinery is liable to be out of order,—there are no cocks to be turned, no valves to be regulated; nor can the operator derange the apparatus but by the most violent efforts. And when the stock of gas is prepared, we may depend on its lighting power as much[154] as we depend on the light to be obtained from a certain number of candles or oil-lamps.
The diversified experiments which have been made by different individuals, unconnected with each other, have sufficiently established the perfect safety of the new lights; and numerous manufactories might be named in which the gas-lights have now been in use for upwards of seven years, where nothing like an accident has occurred, though the apparatus in all of them is entrusted to the most ignorant man.
It would be easy to state the causes which have given rise to some of those accidents that have spread alarm amongst the public; but of this it is not my business to speak at length. It is sufficient, on the present occasion, to state, that those melancholy occurrences which have happened at some gas-light establishments which I have had an opportunity of examining, were totally occasioned by egregious failures committed in the construction of the machinery. Thus, an explosion very lately took place in a manufactory lighted with coal-gas, in consequence of a large quantity of gas escaping into a[155] building, where it mingled with common air, and was set on fire by the approach of a lighted candle. That such an accident could happen, is an evident proof that the machinery was erected by a bungler, unacquainted with the most essential principles of this art; because such an accident might have been effectually prevented, by adapting a waste pipe to the gasometer and gasometer house. By this means, if more gas had been prepared than the gasometer would contain, the superfluous quantity could never have accumulated, but would have been transported out of the building into the open air, in as an effectual manner as the waste-pipe of a water cistern conveys away the superfluous quantity of water, when the cistern is full. Such an expedient did not form part of the machinery.
Other instances might be named, where explosions have been occasioned through egregious mistakes having been committed in the erection of the gas-light machinery, were this a subject on which I meant to treat.
That the coal-gas, when mixed with a certain portion of common air, in close vessels, may be inflamed by the contact of a lighted[156] body, as has been stated, page 98, is a fact sufficiently known. But the means of preventing such an occurrence in the common application of gas-lights, are so simple, easy, and effectual, that it would be ridiculous to dread danger where there is nothing to be apprehended. In speaking thus of the safety of the gas-light illumination, I do not mean to deny that no possible circumstances may occur where the coal-gas may be the cause of accident. It is certain that the gas, when suffered to accumulate in large quantities in close and confined places, where there is no current of air, such as in cellars, vaults, &c. and where it can mix with common air, and remain undisturbed, that it may be liable to take fire when approached by a lighted body; but I do not see how it is probable that such an accumulation of gas should take place in the apartments of dwelling houses. The constant current of air which passes continually through the rooms, is sufficient to prevent the possibility of such an accumulation ever to take place. And with regard to the bursting of the pipes which convey the gas, no accident can possibly happen from that quarter; because the[157] gas which passes through the whole range of pipes sustains a pressure equal to the perpendicular weight of about one inch of water only, and such a weight of course is insufficient to burst iron pipes. Nor could the town when illuminated by gas-lights, be thrown suddenly into darkness, as has been asserted might happen by the fracture of a main pipe, supposing such an event should take place; because the lateral branches, which supply the street-lamps and houses, are supplied by more than one main; and the consequence of a fracture would be only an extinction of the few lamps in the immediate vicinity of the broken pipe, because the rest of the pipes, situated beyond the fracture, would continue to be supplied with gas from the other mains, as will become obvious from the sketch exhibited in the next page.
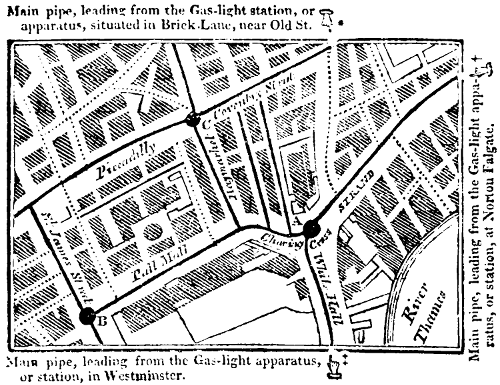
* The gasometer at this place is equal in capacity to 22000 cubic feet.
† The capacity of the gasometer here is equal to 15928 cubic feet.
‡ At this station the gasometer is equal in capacity to 14808 cubic feet.
The black lines represent the gas-light mains, or largest pipes, from which the smaller pipes branch off: they are connected with each other at the places marked A B C; and the dotted lines represent the smaller mains, or collateral branches before-mentioned. The main pipes are all furnished with valves, or cocks, placed at about 100 feet distant from each other. Now let us suppose that a main pipe, in any part of the street marked in the sketch, Pall Mall, should break, it is evident, on mere inspection, that the gas which is[159] passing through the main in the Strand, and which is also connected with the main in the Haymarket, Piccadilly, and Coventry Street, would continue to supply the broken pipe, and the valve nearest to the fracture being shut, would prevent the loss of any considerable quantity of gas, and the few lamps situated between the two valves and the fracture would therefore only become extinguished.
Further, let us suppose a main pipe should break in Piccadilly; in that case, the valve being shut on each side of the fracture, the gas would be supplied from the mains in the Haymarket and St. James’s Street. And the same effect would be produced in any part of the town, supplied with gas-pipes. Besides all this, in the statement thus far given, we have assumed that all the gas-light mains are supplied with gas from one manufacturing station only, but which in reality is not the case. The range of pipes that convey the gas is connected with three gas-light establishments, situated at different parts of the town; and the gas which is supplied from these stations is connected with the whole system of pipes in the[160] streets.[37] If, therefore, one of the manufactories should be annihilated, it would make no difference, because the lights would be amply supplied from the other two manufacturing stations. Hence it is obvious, that the fracture of any of the gas-light mains, or even the total destruction of one or more of the manufactories themselves, would be attended with no serious consequence; and as the system of lighting with gas becomes more extended, the manufactories, or stations for supplying it, will also be multiplied, to give effect and security to the whole.
In fact, no danger can arise from the application of gas-lights in any way, but what is common to candle-light, and lamps of all kinds, and is the fault of none of them. Even in this case the gas-lights are less hazardous. There is no risk of those accidents which often happen from the guttering or burning down of candles, or from carelessly snuffing them. The gas-light lamps and burners must necessarily be fixed to one place, and therefore cannot fall, or otherwise become[161] deranged, without being immediately extinguished. Besides, the gas-light flames emit no sparks, nor are any embers detached from them. As a proof of the comparative safety of the gas-lights, it need only be stated, that the Fire-offices engage themselves to insure cotton-mills, and other public works, at a less premium, where gas-lights are used, than in the case of any other lights.[38] The excessive expence of insurance arising from the numerous candles employed in most of the first rate manufactories, and the combustible nature of the structure of the buildings; the great difficulty of retrieving the injury resulting to a well-organised business, from the accidental destruction of the machinery, are objects alone sufficient to furnish the strongest economical,[162] as well as political recommendations, for the adoption of the new lights in all manufactories where work is done by candle-light.
[38] Since the preceding pages have been printed, I have seen a self-extinguishing gas-lamp, invented by Mr. Clegg. This lamp is so constructed, that the gas cannot flow to the burner, when the flame becomes extinguished. If, therefore, the lamp should be blown out, and the stop-cock which supplies the gas be left open, the extinction of the flame will effectually shut the valve. The action of this lamp depends upon the expansibility of a metallic rod, heated by the flame of the lamp, and thus keeping open the valve, whereas, when the lamp is extinguished, and the rod becomes cold, it contracts to its natural dimensions, and, by that means, effectually closes the valve. The same engineer has invented a machine, which both measures and registers, in the absence of the observer, the quantity of gas delivered by any pipe communicating with a gas-light main. The machine occupies a space of about two feet by one foot, and, if put up in a room, house, or other place, where gas is burnt, will, at any time, by mere inspection, give an account of the quantity of gas consumed in that place during any given time. On the present occasion, it would not become me to say more on these subjects, which, no doubt, Mr. Clegg will make known to the public; I shall only remark, that these contrivances do signal honour to the talents and abilities of the inventor; and that they will render the greatest services to those who are engaged in the gas-light illumination.
After considering the facts so far detailed, many other advantages, connected with the gas-light illumination, will naturally suggest themselves to the reader. I have endeavoured merely to point out the leading characters of the new lights, as they are at present. Ingenious men may speculate from what has been done to what remains to be effected, which, no doubt, will embrace objects of the greatest utility and most extended national importance. The public attention is awakened to the new properties of coal, and will not rest till they are extensively applied to economical purposes. The consequence will be, a considerable[163] defalcation in the revenue. For, in proportion as the gas-lights are more or less generally adopted in all towns of the country, the consumption of oil and tallow will be diminished, and the impost on those articles become less productive; and when this takes place, Government, no doubt, will share in the profits, by levying a tax on the new lights. The Exchequer will thus have nothing to fear; as one branch of the revenue fails, another, and a more productive one, will supply its place.
Upon the whole, when we reflect that the object of the gas-light illumination is to open a source of national wealth, of which nothing can deprive us, to create, we may almost say, new articles of value, its friends cannot be thought guilty of great presumption, if they look forward with confidence to the successful extension of this new art of civil economy; and if, contrary to all expectations, the effects of jealousy and prejudice should, in some respect or other, continue here and there its influence against this new art of procuring light, a firm perseverance of its application must at length remove that ignorance which alone can give them birth.
EXHIBITING
The quantity of Gas, Coke, Tar, Pitch, Essential Oil, and Ammoniacal Liquor, obtainable from a given quantity of Coal; together with an Estimate of the quantity of Coal necessary to produce a quantity of Gas, capable of yielding a Light equal in duration of time and intensity to that produced by Tallow Candles of different kinds.
| Cost of Coal. | Weight of Coal. | Produce of Gas, in cubic feet. | - | [39]Equal to as many tallow candles, 12 in the pound, burning two hours; or to | Candles. | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mini- mum. |
Maxi- mum. |
Aver- age. |
Min. | Max. | Aver. | Min. | Max. | Aver. | 9,516 8,651 |
11 10 |
to the pound. to thdo. |
|||||||||||||||||||||||||
| One Chal. of Coal, from 25 to 28 cwt. |
- | 40 | s | to | 60 | s | — | 50 | s | 2,800 | to | 3,136 | — | 2,968 | 8,906 | to | 11,872 | — | 10,388 | [39] | 7,786 6,921 6,556 5,194 |
10 9 8 7 |
to thdo. to thdo. to thdo. to thdo. |
|||||||||||||
| One Ton | 30 | s | to | 48 | s | — | 38 | s | 6 | d | 2,240 | 6,720 | to | 8,960 | — | 7,840 | 4,325 | 5 | to thdo. | |||||||||||||||||
| One Sack | 3 | s | 4 | d | to | 5 | s | — | 4 | s | 2 | d | 233 | to | 261 | — | 247 | 741 | to | 988 | — | 814 | 3,463 | 4 | to thdo. | |||||||||||
| One Bushel | 1 | s | 2 | d | to | 1 | s | 8 | d | — | 1 | s | 5 | d | 78 | to | 87 | — | 82 | 1⁄2 | 247 | to | 330 | — | 290 | 2,595 | 3 | to thdo. | ||||||||
| One Peck | 3 | 1⁄2 | to | 5 | d | — | 4 | 1⁄4 | 19 | 1⁄2 | to | 21 | 1⁄4 | — | 20 | 1⁄4 | 61 | to | 82 | — | 71 | 1⁄2 | 1,730 | 2 | to thdo. | |||||||||||
| One Pound | 1⁄4 | 1 | 3 | to | 4 | — | 3 | 1⁄2 | 866 | 1 | to thdo. | |||||||||||||||||||||||||
| Coke.—One chaldron of coal, from 25 to 28 cwt. gives 11⁄4 to 1½ chaldron of Coke. | ||||||||||||||||||||||||||||||||||||
| Tar.—One chaldron of coal, from 25 to 28 cwt. gives from 150 to 180lb. of Tar,[39] or 15 to 18 ale gallons, 10lb. each. | ||||||||||||||||||||||||||||||||||||
| Ammoniacal Liquor.—One chaldron of coal, gives from 220 to 240lb. of Ammoniacal Liquor, or 22 to 24 ale gallons. | ||||||||||||||||||||||||||||||||||||
[39]1000lb. of Coal-Tar afford by distillation, from 260 to 265lb. of Essential Oil, or Naphtha. 1000lb. of Coal-Tar produce by mere evaporation, from 460 to 480lb. of Pitch.
Tabular View, exhibiting the illuminating power of Coal-Gas, compared with the illuminating power of Tallow Candles of different sizes.
| One chaldron of Coal produces, according to weight and quality, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Burning. | Candles. | 12 to 1lb. | 6 to 1lb. | |||||||
| Cubic feet of Gas. | Average. | 1 | hour | = | 21,000 | = | 10,500 | |||
| From | 9,000 | to | 12,000 | 10,500 | 2 | hours | = | 10,500 | = | 5,250 |
| 6,000 | 8,000 | 7,000 | 3 | ditto | = | 7,000 | = | 3,500 | ||
| 4,500 | 6,000 | 5,250 | 4 | ditto | = | 5,250 | = | 2,625 | ||
| 3,600 | 4,800 | 4,400 | 5 | ditto | = | 4,400 | = | 2,200 | ||
| 3,000 | 4,000 | 3,500 | 6 | ditto | = | 3,500 | = | 1,750 | ||
| 2,571 | 3,428 | 3,005 | 7 | ditto | = | 3,005 | = | 1,502 | ||
| 2,250 | 3,000 | 2,625 | 8 | ditto | = | 2,625 | = | 1,312 | ||
| 2,000 | 2,666 | 2,333 | 9 | ditto | = | 2,333 | = | 1,166 | ||
| 1,800 | 2,100 | 2,100 | 10 | ditto | = | 2,100 | = | 1,050 | ||
| 1,636 | 2,191 | 1,913 | 11 | ditto | = | 1,913 | = | 956 | ||
| 1,500 | 2,000 | 1,750 | 12 | ditto | = | 1,750 | = | 875 | ||
| 1,384 | 1,846 | 1,615 | 13 | ditto | = | 1,615 | = | 807 | ||
| 1,285 | 1,714 | 1,499 | 14 | ditto | = | 1,499 | = | 749 | ||
| 1,200 | 1,600 | 1,400 | 15 | ditto | = | 1,400 | = | 700 | ||
| 1,125 | 1,500 | 1,312 | 16 | ditto | = | 1,312 | = | 656 | ||
| 1,058 | 1,111 | 1,234 | 17 | ditto | = | 1,234 | = | 617 | ||
| 1,000 | 1,333 | 1,166 | 18 | ditto | = | 1,166 | = | 583 | ||
| 947 | 1,263 | 1,105 | 19 | ditto | = | 1,105 | = | 552 | ||
| 900 | 1,200 | 1,050 | 20 | ditto | = | 1,050 | = | 525 | ||
| 857 | 1,143 | 1,000 | 21 | ditto | = | 1,000 | = | 500 | ||
| 818 | 1,095 | 956 | 22 | ditto | = | 956 | = | 478 | ||
| 783 | 1,044 | 913 | 23 | ditto | = | 913 | = | 456 | ||
| 750 | 1,000 | 875 | 21 | ditto | = | 875 | = | 437 | ||
N. B. If it be required to know, for how many hours one pound, or one peck, or one bushel, or one sack, of coal will produce Gas Light equal to that of a certain number of well-snuffed Tallow Candles, the proportion of each of the average weights of a pound, peck, bushel, or sack, to that of the average weight of a chaldron of coal, is as follows:
| 1 | lb. | = | 2968th | part of a chaldron. | |
| One peck | 20 | = | 148th | ditto. | |
| One bushel | 82 | = | 36th | ditto. | |
| One sack | 248 | = | 12th | ditto. |
Rule.—Divide with either of the above parts of weight, the number of lights opposite to their hours, and the product will be the number of lights burning for the same number of hours.
Example.—To know how many lights one peck of coal will give for six hours, divide the 148th part in 3,500, opposite to the number of six hours, the product is almost 24 lights. The same rule holds good for any given quantity or number of pounds of coal, in a chaldron, to find how many lights, or candles, 12 to the lb. or 6 to the lb. they will give for a given number of hours.
Exhibits a perspective view of a gas-light apparatus,[40] for lighting factories, or small districts of houses. It consists of the following parts: which may be considered separately.
[40] This apparatus was erected by Mr. Clegg, and is now in action at Mr. Ackerman’s establishment, in this metropolis.
Fig. 1. The Retort Furnace, for distilling the coals. It is built of brick-work. The bricks which are exposed to the immediate action of the fire, are Welch tumps, or fire-bricks; they are bedded in clay, or Windsor loam.
Fig. 2. The Tar Cistern, to collect the coal-tar, and other condensible products obtained during the distillation of the coals. It is a cast-iron hollow cylinder, closed at the top with a cast-iron cover, which has a very small hole to allow the air to escape as the liquid enters into the vessel.
Fig. 3. The Lime Machine, for purifying the crude coal-gas, and to render it fit for use. The construction of this machine will be explained in plate VII. It is put together of cast-iron plates.
Fig. 4. The Gasometer, for collecting and preserving the purified gas, and for distributing and applying it as occasion may require. It consists of two principal parts—namely, a large interior vessel closed at the top and open at the bottom, made of sheet iron, designed to contain the gas, and an outer cistern or vessel, of rather greater capacity, constructed of cast-iron plates, in which the former vessel is suspended. The latter contains the water by which the gas is confined. The interior vessel which contains the gas is suspended by chains hung over wheels or pullies, to which weights are attached, so as to be just sufficient to balance the weight of the gasometer, all but a small difference, and allowing its slow descent in the manner which is found as nearly adapted as can be to the proper supply of the lamps. The weight of the chains must be equal to the specific gravity of the material of which the gasometer is composed, so as to compensate accurately for the quantity of water which the gasometer displaces, or what is the same, it must be equal to the loss of weight which the gasometer sustains, when immersed in the water; and the counterpoise weight must be equal (or nearly so) to the absolute weight of the gasometer.
The action of these different parts of the apparatus will be obvious from the following explanation:
A, A, are two iron retorts, placed horizontally, and side by side, in the furnace; the mouth of the[168] retorts where the coals are introduced, projects into an arched chamber, situated in front of the furnace, as shewn in the drawing by the broken down brick-work. The object of suffering the mouth of the retorts to project into a separate chamber, is merely to discharge with convenience the red hot coke from the retorts when the process is at an end; the coke being suffered to fall to the bottom of the chamber, where it cools, without becoming troublesome to the operator. It may be removed from this fire-safe chamber by the door represented at the end view of the furnace.
When the operation commences, the inner vessel of the gasometer, fig. 4 is sunk down, to expel the air which it contains to a level with the exterior vessel, or outer cistern, of the gasometer; and, consequently, becomes filled with water. As the distillation of the coal in the retorts proceeds, the liquid and gazeous products evolved from the coals are transmitted by means of the perpendicular syphon pipes B, B, into the horizontal pipe or main condenser C, with which they are connected. The liquid which is distilled, collects in the pipe, or main condenser, C, where it is retained until its quantity has risen so high as to discharge itself into the pipe D, which is connected with the upper part of one of the extremities of the condenser, C. One of the extremities of the pipes, B, B, therefore become immersed into the liquid contained in the main condenser or pipe C, whilst the vaporous or condensible fluid, after having overcome the pressure there opposed to it, is transported into the pipe E, which, after passing in a serpentine direction, E, E,[169] &c. through the exterior vessel or cistern of the gasometer, terminates in the tar-vessel, fig. 2. Thus the vaporous fluids are condensed by passing through the serpentine pipe, E, E, &c. and become deposited in the tar-cistern, fig. 2; whilst the non-condensible or gazeous products are made to proceed by the pipe F, which branches off from the pipe E, into the lime machine, fig. 3. In this apparatus the gas, as it is evolved from the coals, comes into contact with slaked lime and water; the object of which is, to strip it of its sulphuretted hydrogen and carbonic acid gas with which it always abounds, and to render it fit for illumination. This being accomplished, the purified gas is conducted away out of the lime machine by means of the pipe G, into the perpendicular pipe H, which branches up through the bottom of the gasometer cistern. The upper extremity of this pipe is covered, in the manner of a hood, by a cylindrical vessel I, open at bottom, but partially immersed beneath the surface of the water contained in the outer cistern of the gasometer, it is also perforated round near the lower edge with a number of small holes. The gas, as it passes out of the pipe H, displaces the water from the receiver I, and escapes through the small holes, and is thus made to pass through the water in the cistern, in which the hood of the pipe I, is partly immersed, so as to expose a large surface to its action, that it may once more be washed, and deprived of all the foreign gazeous products which might have escaped the action of the lime, whilst it was agitated[170] with this substance in the lime machine, fig. 3. After rising through the water in the gasometer cistern, it enters into the gasometer, which then ascends as the gas accumulates in it.
In this manner the process proceeds, until the whole of the volatile products of the coal in the retort are disengaged. The use of the gasometer is, partly to equalize the evolution of the gas which comes from the retort more quickly at some time than others. When this happens, the vessel rises up to receive it, and when the stream from the retort diminishes, the weight of the gasometer expels its contents, provided the main-cock be open. When the process is finished, the retort is suffered to cool, and its lid is then removed to replenish it with coal. When the main stop-cock is then opened, the gasometer descends, and the gas passes from the gasometer through the pipe K, to the burners, or main pipe, which communicates with the gas burners or lamps. L, is a wooden tub or barrel, containing the mixture of lime and water, for charging the lime machine; and into which the contents of the barrel, L, may be conveyed by the curved pipe M, without admitting common air. N, N, is a water-pipe, to convey fresh water into the gasometer cistern occasionally; because it is essential that the water used for washing and purifying the gas should be changed for fresh as soon as it becomes dirty; and unless this is done, the gas will not be perfectly purified by washing, but produce a disagreeable odour when burnt; the same holds good with regard to the lime[171] machine, the contents of which should be renewed occasionally. This pipe also conveys the necessary water into the barrel, L. O, is a waste-pipe, to convey the water as it becomes impregnated with the impurities of the gas, out of the gasometer cistern. P, is an agitator, to stir up the contents of the lime machine occasionally, Q, Q, are two iron rods, which serve as stays to guide the motion of the gasometer. R, is an index, connected by means of a shaft and pulley with the axis of one of the gasometer wheels. This index is graduated to the capacity of the cubical contents of the gasometer, so as to indicate, by the rising and falling of the gasometer, its relative contents of gas expressed in cubic feet. S, is the waste pipe of the lime machine, to remove the insoluble parts of the lime. T, represents the iron cover, or lid, which is turned on the lathe, and ground air-tight, to close up the mouth of the retort, so as to make readily an air-tight fitting. U is an iron wedge to secure the cover of the retort. The left-hand retort in the design shows the retort closed up, and the cover, or lid of the mouth of it secured by means of the wedge, in its place, so as to render the mouth of the retort perfectly air tight.
There is a safety valve attached to this gasometer which could not be represented in the drawing; and the object of which is, to convey away any portion of gas that might happen to be produced by a careless operator, when the gasometer is full, and which is thus prevented from accumulating in the place where the gasometer is erected. It is represented in[172] the right-hand corner of plate VII. where fig. 1 shows the edge of the gasometer; 2, the surface of the water in the inside of the gasometer; 3, the surface of the water in the outside of the gasometer, or in the cistern; 4, a pipe issuing from the lower edge of the gasometer, and surrounded at its upper extremity with a cup marked 5; 6, the waste pipe, the mouth of which is immersed in water. It is obvious that, when the gasometer is full, if an additional quantity of gas should be attempted to be put into it, it will be transported by means of the pipe 4, into the waste-pipe 6; the upper extremity of which reaches out of the building, and there communicates with the open air.
Represents a Portable experimental Gas Apparatus for exhibiting, in the small way, the general nature of the gas-light illumination.—It is described page 79.
Show designs of various kinds of Gas Lamps, Chandeliers, Candelabras, &c.—See pages 114, 118, 140.
Fig. 1. Exhibits a design of the gasometer framing, or skeleton, which serves to give stability and strength to the gasometer. It consists of wooden frame work, marked A, A, A, interlaced[173] with iron rods, B, B, B, &c. The whole framing is so disposed that it will float in the cistern horizontally, and therefore keep the gasometer perfectly steady and level with the surface of the water.
The rest of the sketches represent various kinds of gas pipes employed as mains for conveying the gas, and the methods of connecting them.
Fig. 2. Represents a longitudinal section of a Spigot and Faucet Pipe. These kinds of pipes are applicable in most cases as mains for conveying gas. A, is called the spigot, and B, the faucet. They are joined together, and made air tight, by iron cement, the composition of which is as follows:
Take two ounces of sal ammoniac, one ounce of flowers of sulphur, and sixteen ounces of cast iron filings or borings. Mix all well together, by rubbing them in a mortar, and keep the powder dry.
When the cement is wanted for use, take one part of the above powder, and twenty parts of clean iron borings or filings, and blend them intimately by grinding them in a mortar. Wet the compound with water, and when brought to a convenient consistence, apply it to the joints with a wooden or blunt iron spatula.
By a play of affinities, which those who are at all acquainted with chemistry will be at no loss to comprehend, a degree of action and re-action takes place among the ingredients, and between them and the iron surfaces, which at last causes the whole to unite as one mass. In fact, after a time, the mixture and the surfaces of the flanches become a species of pyrites (holding a very large[174] proportion of iron,) all the parts of which cohere strongly together.
The inner parts of the faucet ought to be no larger in diameter than just to fit the spigot. This supports the pipe, independently of the cement, and prevents the risk of hurting the joint from any external stress. The inner faucet is commonly made about 21⁄2 inches deep, and has the spigot inserted 11⁄2 inch into it. The practice of some workmen, is to make the outer faucet, or that which contains the cement, six inches deep, for all pipes above six inches diameter; and to make the faucets of all pipes below six inches, the same depth as the diameter of the pipes. It is usual to make the space for the cement, all round the spigot, from 1 to 11⁄2 inch; that width is required, in order that the cement may be firmly driven into the joint. When the space is very narrow, this cannot be done. On the other hand, when too wide, there is a waste of cement, and a risk of injury from unequal expansion.
Fig. 3. Exhibits a profile view of these kinds of pipes when joined together. The spigot and faucet pipes are liable to burst from the great expansion of the spigot, and the risk of this accident is increased by increasing the space between the spigot and faucet, which requires to be filled with cement.
Fig. 4. Represents a longitudinal section of two flanch pipes, and the modes of connecting them. A and B, show the parts of the pipes; and C and D, the flanches. These pipes are also joined together, and rendered air-tight, by interposing between[175] the flanches rope-yarn, hemp, or some other pliable material, and iron cement, and then screwing up the faces of them by means of the bolts and screw nuts.
Fig. 5. Profile view of the same kind of pipes connected together, A and B, the pipes; C and D, the flanches; E and F, the bolts.
Fig. 6. Represents the method of joining spigot and faucet pipes when they are to have a turn or angle. This method is convenient when the place where the turn required to be made is previously known, and the pipes cast accordingly.
Fig. 7. Exhibits the method of connecting spigot and faucet pipes when they have a round turn. A and B, the junctures of the pipes.
Fig. 8. Represents a longitudinal section of the mode of joining pipes by means of what is called a thimble joint. The junctures of the pipes to be connected, are made air tight, as mentioned already, by iron cement. A, the thimble or small cylinder, with projecting edges, which unites the pipes B, C.
Fig. 9. A thimble joint made in two parts, which is sometimes convenient to join pipes. The parts are joined together by screw bolts, and nuts, in the usual manner.
Fig. 10. Section of the same.
Fig. 11. Represents a profile view of what is called the saddle joint. It is employed for taking off a branch-pipe. The branch has a piece A B, formed on its end, and fits round one-half of the outside of the pipe from which it is to proceed. C, is called the saddle, which fits round the other half of the pipe. The parts are secured together[176] by screw bolts, and iron cement. By this method a branch may be formed on any part of a gas-pipe, by cutting a hole there, and applying the branch to that place. Where there is much risk of the inequality of expansion, the joints at certain places, should be secured by a soft stuffing of hemp and tallow; but in most cases the joints may be made with iron cement. Lead is frequently used for making the joints of gas pipes instead of iron cement, though cheaper and more easy of repair. The galvanic action which takes place between the lead and iron, soon renders the joints leaky, and the danger is increased by the unequal expansion of the two metals.
Fig. 12. Section of the saddle-joint.
Before the gas is suffered to enter into the pipe, they should be proved to be sound, by the usual process of forcing water into them: The pipes serving as mains, are placed perfectly solid, so that they cannot give way; their course should be rectilinear, having a descent of about 1 inch in 9 or 10 feet, to allow the water of condensation which may be deposited from the gas by a change of temperature to collect readily at the lowermost part.
Fig. 13. Shows a reservoir for collecting the water of condensation which might accumulate in the pipes. It consists of a receptacle, A, in which the water may pass; B, a branch-pipe closed at the top, by means of which the water may be removed, by drawing it out with a syringe. This receptacle is placed in those situations where pipes incline towards each other.
Exhibits a perpendicular section of a gas-light apparatus, calculated for lighting towns, or large districts of streets and houses.
Fig. 1. The Retort Furnace. The retorts are placed over each other in one or more rows; so that a certain number of them may be heated by separate fire-places. A, A, shows two of the retorts placed horizontally above each other; B, the fire-place; C, the flue which causes the fire to circulate round the retorts so as to heat them equally in every part; D, the opening of the flue where the fire passes into the chimney; E, the ash-pit; F, a chamber in front of the retort furnace, into which the orifice or mouth of the retorts project; G, G, the doors of the chamber, to enable the workmen to charge and discharge the retorts; H, a funnel shaped hole at the floor of the chamber F, through which the red hot coke as it is discharged from the retorts passes into the arched vault I; K, the syphon tube; L, the horizontal condenser[41]—the action of both of these pipes have been already explained, p. 168; M, main pipe, which conveys the liquid substances from the condenser, to the tar cistern, fig. 3, and[178] which conducts also the gazeous products into the lime machine, fig. 2; N N, shows that part of the pipe which is interposed between the tar cistern, fig. 3, and the condensing pipe M,—it passes in a serpentine direction along the inner sides of the gasometer cistern, and, like the so-called worm in a distillatory apparatus, condenses the products which escape in a vaporous state from the condenser L; O, shows the place where the serpentine pipe N N, passes again out of the gasometer cistern, and its communication with the lime machine, fig. 2, and tar chamber, fig. 3. The action of the lime machine is as follows: The liquid products evolved from the coal, having been deposited in the tar cistern, fig. 3, by means of the serpentine pipe N, N, the gazeous products which accompany it, are conveyed by means of the pipe P, which branches out from the pipe O, into the interior receptacle of the lime machine marked Q, which consists of a vessel open at the bottom, and closed at the top, where it communicates with the pipe O. As the gas accumulates in the interior part Q, of the lime machine, it is made to pass through the liquid which it contains, namely, slaked lime and water; and escapes through appertures made in the horizontal partitions R, R, R, R, into the outer vessel, S, of the lime machine and from thence it is conducted away by the pipe T, T, T, into the additional washing apparatus, of the gasometer; fig. 4, the construction of this apparatus, greatly resembles the lime machine, fig. 2, namely, V, is a water pipe, proceeding from a cistern U, placed 3 or 4 feet above the orifice of the pipe V; T, T, is the gas-pipe,[179] covered with a hood, marked W, and immersed in a small cistern, having horizontal perforated shelves, like those in the lime machine—they fit close to the hood. The gas which enters the hood W, meets with a shower of water delivered by the pipe V. The gas, as it passes through the holes in the horizontal partitions, is, therefore, again washed and thoroughly purified from foreign gases which may have escaped the action of the lime machine; Y, is a waste pipe, the lower extremity of which is sealed by being immersed in water,—it serves to carry away the water delivered by the pipe V, as it has been acted on by the gas. The summary action of this gas apparatus is, therefore, as follows: The liquid products obtained from the coal during the distillation are first deposited in the main condenser L, by means of the pipe K, and from whence they cannot escape until a quantity of tar has accumulated in it to a certain height, and by this means, one of the extremities of the pipes K, K, becomes immersed and hermetically sealed by the liquid which the condenser L, contains. The liquid products, after having accumulated to a certain height in the condenser, overflow the perpendicular portion which it contains, and discharge themselves into the pipe M, from whence they are transported into the tar cistern, fig. 3, by means of the system of pipes N, N, O, whilst the gazeous products are made to pass by means of the branch pipe P, into the lime machine, fig. 2. From this part of the apparatus the gas passes through the pipe T, T, T, into the additional or smaller washing apparatus placed upon a tressel in the cistern of the[180] gasometer, where it is again exposed a second time to the action of a current of fresh water; and from this vessel the gas ascends into the gasometer. The gasometer is furnished with a pipe A, closed at the top, and fixed in one corner of the gasometer, but open at the bottom; it includes another pipe marked B, which communicates with the main pipe leading to the burners, or place where the gas is wanted. The pipe A, which slides over the pipe B, is perforated at the top, the gas passes through these perforations and is thus made to enter into the pipe B, and disposed of as mentioned. C, C, is a tube of safety adapted to the gasometer; its lower extremity remains sealed by the water in the cistern so long as the gasometer is not overcharged with gas; but, if more gas should be made to enter the gasometer than it is destined to receive, this pipe then delivers the gas into the funnel-shaped tube D, which reaches through the roof of the gasometer house, and thus the superfluous quantity of gas is conveyed away into the open air.
[41] The condenser in this apparatus is placed at right angles to the row, or rows of retorts. It is furnished at one extremity with a partition placed perpendicularly, and of a height equal to about one-half of the diameter of the condenser. The object of this partition is to prevent the tar, &c. deposited in it, to seal the pipes K, K, and not to discharge itself into the pipe M, till this has been effected. The partition is seen in the drawing.
The cylindrical vessel P, of fig. 3, surrounding the orifice of the pipe O, which delivers the tar into the tar cistern, fig. 3, serves to keep this pipe constantly immersed into a portion of tar, so that the contents of the cistern may be drawn off by the cock without admitting air into any part of the apparatus. The tar cistern has a small hole at the top, to allow the air which it encloses to escape, as it becomes filled with tar and ammoniacal liquor. The main condenser L, is placed, as shown in the drawing, higher than the level of the water in the gasometer cistern,[181] to allow a free descent of the distillatory liquids as they pass from this vessel along into the pipes M, N, O, &c. The cistern of the gasometer, as well as the lime machine, and tar cistern, are constructed of cast iron plates, bolted and cemented together with iron cement. The gasometer is made of sheet iron plates rivetted together—E, E, are two iron stays—G, G, are friction wheels.
[42] For this elegant contrivance we are also indebted to Mr. Clegg.
We have mentioned already that the pressure of the gas in the gasometer should be invariable, for it is obvious that the weight of the gasometer is constantly increasing in proportion as it fills with gas, and rises out of the water—see p. 88, and 167. To render its pressure uniform, we first take the absolute weight of that part of the gasometer which becomes immersed in the water, and knowing the specific weight of the substance of which it is composed, we divide its absolute weight by the specific weight of the substance of which it is composed; and this being done, we make part of the chain, (measured at right angles from the axis of the wheels over which it passes downwards towards the top of the gasometer,) which is equal to the length of that part of the gasometer which becomes immersed in water, equal in weight to the specific gravity of the substance[182] of which the gasometer is composed. For example, let us suppose that the part of the gasometer which becomes immersed in water weighs 861 lb. and that it is composed of sheet iron, the specific gravity of which, in round numbers, we will take to be 7. It is then evident, that the part of the chain of the gasometer measured downward from the axis of the wheel over which it passes, and which is equal in length to the height of the gasometer, must be loaded with a weight of, or must itself weigh, 123lb. for this would be the weight of the water displaced by the gasometer; or let us suppose the gasometer to be made of sheet copper, the specific weight of which (omitting decimals) is 8; and that the absolute weight of the gasometer is 1792lbs. then the chain of the gasometer equal in length to the height of the gasometer, immersed into the water must weigh 224lb. for this would be the weight of the quantity of water which the gasometer displaces. This being accomplished by then adding or diminishing the absolute or balance weight of the gasometer, any desired uniform pressure may be effected, and the same bulk of gas will always be of the same specific gravity.
[43] Copied from a printed direction drawn up by Mr. Clegg, for the use of workmen.
Particular care must be taken to make the joints of the mouth-pieces of the retorts perfectly air tight, which may be done in the following manner:—Take[183] some common clay, dry, pulverize, and sift it, then add as much water as will make it into the consistency of treacle; make the mouth-piece and the lid of the retort clean, lay this luting thinly over the turned part of the lid, press the lid so luted gently to the mouth-piece, and then secure it moderately, by means of the iron wedge: if the workman observes this rule, he will never fail to make good joints; but if, on the other hand, the operator is careless and neglects to remove the old luting, &c. from the turned or smooth part of the mouth of the retort, and thereby cause a bad joint, the consequence will be the loss of a considerable quantity of gas, and a very disagreeable smell and smoke.
The bridge or row of bricks of the flue C, of the retorts, should never be made hotter than a bright red, which may be regulated by the door of the ash-pit being kept close shut when the fire is getting too hot. If the operator neglects this, and suffers the fire-bricks to arrive at a bright white heat the retorts will soon be destroyed, and bad gas be produced.
The gasometer should be well examined, at least once a week, to see if it leaks, by the following method, viz. Let the main stop-cock be shut, then make a mark on the gasometer at the water’s edge when it is full or nearly of gas, there being no gas coming from the retorts at the time, and if the mark sinks in the water, the gasometer leaks; to find out the place, walk slowly round it, and you may perceive the leak by the smell, apply a lighted candle to the part suspected, and if there be gas issuing from[184] it, it will take fire, and perhaps appear like a small blue flame—blow it out, and mark the place: thus proceed round the gasometer till you have found all the places; if you perceive a smell, and yet cannot produce a flame in the part suspected, take a brush with a little thin white-lead paint, and lay it on the part where you think the leak is, and, if it be there, the gas which escapes from the leak, will immediately turn the paint brown. After the sides of the gasometer have been well examined, and secured by dipping a piece of cloth about the size of a shilling, into some melted pitch, tempered with a little bees-wax and tar, apply the cloth whilst hot to the place with the end of your finger, rubbing it till it is quite cold; next examine the top of the gasometer in the same manner,—when it is about two feet high in the cistern, it will then be better to get at. The water in the cistern should always be kept within 3 or four inches of the top, if suffered to sink much lower without replenishing, the gas will not pass through a sufficient quantity of water, and oily particles will be apt to condense in the pipes, to their great detriment.
The only thing to be observed in the place lighted is, that the lamps and pipes are not suffered to be touched on any pretence whatever, but by the person entrusted with their care. When a lamp is not wanted, it must be completely shut off from the pipe which supplies it, by a stop-cock provided for the purpose, and not opened again but when a flame is held over it; not a lighted candle, as the tallow is liable to drop into the lamps; lighted paper is better.
Capable of affording, every 24 hours, Light equal to 40,000 Tallow Candles, six in the pound, burning one hour.
| £. | s. | |
|---|---|---|
| Gasometer, to contain 10,000 cubic feet of gas | 236 | 0 |
| Wheel-work, regulating chain, ballance-weight for ditto, with wooden framing | 160 | 11 |
| Wrought iron cistern for gasometer—36 feet wide, 24 feet long and 16 feet deep | 500 | 0 |
| (It would weigh about 16 tons.) | ||
| Wooden framing built around it, to secure ditto | 150 | 0 |
| Condenser, cistern and communicating pipes | 126 | 0 |
| Lime machine, made of cast iron plates | 82 | 0 |
| Gasometer-house, built of frame-work and weather-boarded | 250 | 0 |
| Twenty-four retorts set in brick-work, with furnaces for ditto, compleat | 336 | 0 |
| Sundries | 100 | 0 |
| £ 1940 | 11 | |
A gas-light apparatus complete for work, capable of affording every twenty-four hours a quantity of light equal to 1,400 Argand’s Lamps, each lamp equal in intensity to six candles, six in the pound, burning for five hours, will cost 3,500l. if erected in this metropolis.
[44] All the articles are warranted to be perfect and of the best kind. They are delivered free of expence at any wharf between London and Westminster-bridge.
| Sheet-iron pipes brazed. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| s. | d. | ||||||||
| 1⁄4 | inch in diameter | 0 | 4 | a foot | - | in 15 to 18 feet lengths. |
|||
| 3⁄8 | ditto | 0 | 4 | ditto | |||||
| 1⁄2 | ditto | 0 | 5 | ditto | |||||
| 5⁄8 | ditto | 0 | 6 | ditto | |||||
| 3⁄4 | ditto | 0 | 6 | 1⁄2 | ditto | ||||
| 7⁄8 | ditto | 7 | ditto | ||||||
| 1 | inch, ditto | 0 | 7 | 1⁄2 | ditto | ||||
| 1 | 1⁄4 | ditto | 0 | 9 | ditto | ||||
| 1 | 1⁄2 | ditto | 0 | 10 | 1⁄2 | ditto | |||
| 1 | 3⁄4 | ditto | 0 | 11 | ditto | ||||
| 2 | inch, ditto | 1 | 1 | 1⁄2 | ditto | ||||
| 2 | 1⁄4 | ditto | 1 | 4 | ditto | ||||
| 2 | 1⁄2 | ditto | 1 | 5 | ditto | ||||
| 3 | inch, ditto | 1 | 6 | 1⁄2 | ditto | ||||
| Copper pipes brazed 1⁄4 inch | 0 | 4 | per foot | ||||||
| Ditto, ditto, ditto 3⁄8 inch | 0 | 5 | 1⁄2 | ditto | |||||
| Gas-light cockspur burners with stop-cock 2s 6d to 3s 6d | |||||||||
| Argand’s lamps, with glass-holders, from 3s to 4s 6d | |||||||||
| Cast-iron retorts, weighing 7 cwt. at 15s 6d per cwt | £5 | 8 | 6 |
| Mouth-piece for ditto, compleat | 1 | 14 | 8 |
| Cast-iron door frames for retort furnace | 1 | 0 | 0 |
| Furnace bars 10s. per cwt. | |||
| Sheet iron for gazometer (No. 23) 24s. per cwt. | |||
| Gazometer chains, 5d per lb. | |||
| Ballance weights [Plates] for gazometer, 9l 10s per ton. | |||
| Cast-iron cistern plates | |||
| ----------------------- smaller size for lime machine, 18l per ton. | |||
| ----------------------- middling size for tar cistern, 16l ditto | |||
| ----------------------- largest size for gazometer cistern 14l ditto | |||
| Cast-iron flanch pipes | 2 | - | inch diameter, at | 5s | per yd. in | 6 | feet lengths | ||
| ditto | 3 | ditto | 6s | ditto | 6 | ditto | |||
| ditto | 4 | ditto | 8s | 6d | ditto | 9 | ditto | ||
| ditto | 5 | ditto | 10s | ditto | 9 | ditto | |||
| ditto | 6 | ditto | 12s | ditto | 9 | ditto | |||
| ditto | 7 | ditto | 13s | 6d | ditto | 9 | ditto | ||
| ditto | 8 | - | 11l. 5s. per ton | 9 | ditto | ||||
| ditto | 9 | ||||||||
| ditto | 10 | ||||||||
| ditto | 11 | ||||||||
| 1⁄2 inch nuts, screws and washers to put iron pipes together | 7d. | per lb |
| 5⁄8 ditto | 7d. | ditto |
| 3⁄4 ditto | 6d. | ditto |
| English bar-iron | 13l. | per ton |
| Best, ditto | 18l. | ditto |
FINIS.
The entries in the Table of Contents do not always conform to the chapter and section headings in the text. Both have been retained as in the original work.
The errata have already been incorporated in the text; the error mentioned as occurring on page 24 actually occurs on page 22.
The original language, including inconsistencies in spelling, hyphenation, punctuation, formatting, etc. has been retained, except as mentioned below.
The e-reader cover image has been created for this project, and is placed in the public domain.
Unclear parts of the text have been checked against the on-line copy of this book of the Eidgenössische Technische Hochschule Zürich.
Fractions like 1⁄2 and 1-10th have both been retained.
Page 90, Van Dieman, Troostwyck: Jan Rudolph Deiman and Adriaan Paets van Troostwijk.
Changes made to the text:
Obvious punctuation and typographical errors have been corrected silently.
Some footnotes, tables and illustrations have been moved; some tables have been re-arranged.
Other changes:
Page 23: any surfaces changed to any surface
Page 26: opening or shuting changed to opening or shutting
Page 47: A New changed to A new
Page 48: trafic changed to traffic; footnote [10]: corporated changed to incorporated (cf. errata)
Page 53: This combustion changed to The combustion (cf. errata)
Page 64: Cleg changed to Clegg (cf. errata); footnote anchor [14] moved from next page (cf. errata, footnote anchor *); communicates changed to communicated (cf. errata)
Page 67: 1250 + 2 = 2500 changed to 1250 × 2 = 2500
Page 69: Mr. Lee changed to “Mr. Lee for consistency
Page 72: closing quote mark added to letter
Page 96: pure coal- changed to pure coal-gas
Page 102: sub acetate changed to sub-acetate
Page 118: ball 6 changed to ball b
Page 119: e, are changed to e e, are
Page 125: 180 degree changed to 180 degrees (cf. errata); footnote [28]: may he compleatly changed to may be compleatly
Page 131: and make changed to and makes
Page 132: coal changed to coal-tar (cf. errata)
Page 158: Nortou Falgate changed to Norton Falgate; a about changed to about
Page 165, table: 10,509 changed to 10,500.