
Title: The Cambridge natural history, Vol. 03 (of 10)
Editor: S. F. Harmer
Author: A. H. Cooke
F. R. C. Reed
Editor: Sir A. E. Shipley
Release date: September 20, 2023 [eBook #71693]
Language: English
Original publication: New York: MacMillan and Co
Credits: Peter Becker,Karin Spence and the Online Distributed Proofreading Team at https://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)
Transcriber’s Note:
This work features some large and wide tables. These are best viewed
with a wide screen.
EDITED BY
S. F. HARMER, M.A., Fellow of King’s College, Cambridge; Superintendent
of the University Museum of Zoology
AND
A. E. SHIPLEY, M.A., Fellow of Christ’s College, Cambridge;
University Lecturer on the Morphology of Invertebrates
VOLUME III

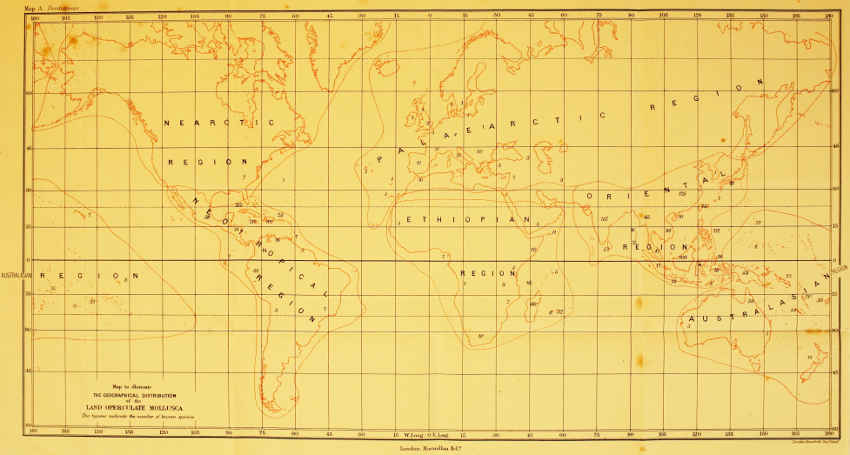
Map to illustrate
THE GEOGRAPHICAL DISTRIBUTION
of the
LAND OPERCULATE MOLLUSCA
The figures indicate the number of known species.
MOLLUSCS
By the Rev. A. H. Cooke, M.A., Fellow and Tutor of King’s College, Cambridge
BRACHIOPODS (RECENT)
By A. E. Shipley, M.A., Fellow of Christ’s College, Cambridge
BRACHIOPODS (FOSSIL)
By F. R. C. Reed, M.A., Trinity College, Cambridge
New York
MACMILLAN AND CO.
AND LONDON
1895
All rights reserved
“Why, you might take to some light study: conchology, now; I always think that must be a light study.”
George Eliot, Middlemarch.
Copyright, 1895,
By MACMILLAN AND CO.
Norwood Press:
J. S. Cushing & Co.—Berwick & Smith.
Norwood, Mass., U.S.A.
[v]
The general plan of classification adopted in this work is not that of any single authority. It has been thought better to adopt the views of recognised leading specialists in the various groups, and thus place before the reader the combined results of recent investigation. This method may, perhaps, occasion a certain number of small discrepancies, but it is believed that the ultimate effect will be to the advantage of the student.
The classification adopted for the recent Cephalopoda is that of Hoyle (‘Challenger’ Reports, Zoology, vol. xvi.), for the fossil Cephalopoda (Nautiloidea) that of Foord (Catalogue of the Fossil Cephalopoda in the British Museum, 1888–91), and (Ammonoidea) P. Fischer (Manuel de Conchyliologie, 1887). In the Gasteropoda the outlines are those adopted by Pelseneer (Mém. Soc. Malacol. Belg. xxvii. 1894), while the details are derived, in the main, from P. Fischer. The Amphineura, however, have not been regarded as a separate class. The grouping of the Nudibranchiata is that of Bergh (Semper, Reisen im Archipel der Philippinen, ii. 3). The Pelecypoda are classified according to Pelseneer’s most recent grouping.
Acknowledgment of the principal sources of information has been made in footnotes, and a short list of leading authorities has been appended to the chapters on anatomy, for the use of students desirous to pursue the subject further. In the case of geographical distribution the authorities are too numerous and scattered to admit of a list being given.
A special word of thanks is due to Mr. Edwin Wilson for his patient care in preparing the illustrations, the majority of which are taken from specimens in the University Museum of Zoology. Mr. Edgar Smith, besides affording the kind help which visitors to the British Museum always experience at his hands, has permitted me to use many specimens for the purposes of illustration.
A. H. COOKE.
King’s College, Cambridge,
20th December 1894.
[vi]
Scheme of the Classification adopted in this Book.
| MOLLUSCA | |
|---|---|
| CHAPTER I | |
| Introduction—Position of Mollusca in the Animal Kingdom—Classification—Origin of Land and Fresh-water Mollusca | 1 |
| CHAPTER II | |
| Land and Fresh-water Mollusca, their Habits and General Economy | 23 |
| CHAPTER III | |
| Enemies of the Mollusca—Means of Defence—Mimicry and Protective Coloration—Parasitic Mollusca—Commensalism—Variation | 56 |
| CHAPTER IV | |
| Uses of Shells for Money, Ornament, and Food—Cultivation of the Oyster, Mussel, and Snail—Snails as Medicine—Prices Given for Shells | 96 |
| CHAPTER V | |
| Reproduction—Deposition of Eggs—Development of the Fertilised Ovum—Differences of Sex—Dioecious and Hermaphrodite Mollusca—Development of Fresh-water Bivalves | 123[vii] |
| CHAPTER VI | |
| Respiration and Circulation—The Mantle | 150 |
| CHAPTER VII | |
| Organs of Sense: Touch, Sight, Smell, Hearing—The Foot—The Nervous System | 177 |
| CHAPTER VIII | |
| The Digestive Organs, Jaw, and Radula: Excretory Organs | 209 |
| CHAPTER IX | |
| The Shell, its Form, Composition, and Growth—Designation of its Various Parts | 244 |
| CHAPTER X | |
| Geographical Distribution of Land and Fresh-water Mollusca—The Palaearctic, Oriental, and Australasian Regions | 277 |
| CHAPTER XI | |
| Geographical Distribution of Land Mollusca (continued)—The Ethiopian, Nearctic, and Neotropical Regions | 328 |
| CHAPTER XII | |
| Distribution of Marine Mollusca—Deep-sea Mollusca and their Characteristics | 360 |
| CHAPTER XIII | |
| Class Cephalopoda | 378[viii] |
| CHAPTER XIV | |
| Class Gasteropoda—Amphineura and Prosobranchiata | 400 |
| CHAPTER XV | |
| Class Gasteropoda (continued): Opisthobranchiata and Pulmonata | 427 |
| CHAPTER XVI | |
| Classes Scaphopoda and Pelecypoda | 444 |
| BRACHIOPODA (RECENT) | |
| CHAPTER XVII | |
| Introduction—Shell—Body—Digestive System—Body Cavity—Circulatory System—Excretory Organs—Muscles—Nervous System—Reproductive System—Embryology—Habits—Distribution—Classification | 463 |
| BRACHIOPODA (FOSSIL) | |
| CHAPTER XVIII | |
| Introduction—Division I. Ecardines—External Characters—Internal Characters—Division II. Testicardines—External Characters—Internal Characters—Synopsis of Families—Stratigraphical Distribution—Phylogeny and Ontogeny | 491 |
[ix]
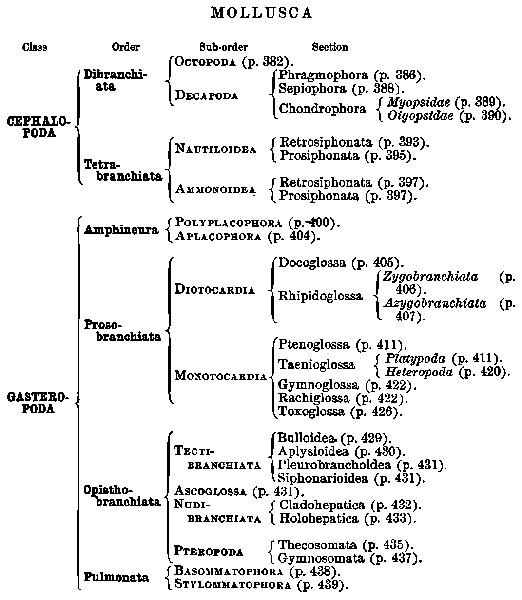
MOLLUSCA
| Class | Order | Sub-order | Section | |
| CEPHALOPODA | Dibranchiata | Octopoda (p. 382). | Phragmophora (p. 386). | |
| Sepiophora (p. 388). | ||||
| Decapoda | Chondrophora | Myopsidae (p. 389). | ||
| Oigopsidae (p. 390). | ||||
| Tetrabranchiata | Nautiloidea | Retrosiphonata (p. 393). | ||
| Prosiphonata (p. 395). | ||||
| Ammonoidea | Retrosiphonata (p. 397). | |||
| Prosiphonata (p. 397). | ||||
| GASTEROPODA | Amphineura | Polyplacophora (p. 400). | ||
| Aplacophora (p. 404). | ||||
| Prosobranchiata | Diotocardia | Docoglossa (p. 405). | ||
| Rhipidoglossa | Zygobranchiata (p. 406). | |||
| Azygobranchiata (p. 407). | ||||
| Monotocardia | Ptenoglossa (p. 411). | |||
| Taenioglossa | Platypoda (p. 411). | |||
| Heteropoda (p. 420). | ||||
| Taenioglossa | ||||
| Gymnoglossa (p. 422). | ||||
| Toxoglossa (p. 426). | ||||
| Tectibranchiata | Bulloidea (p. 429). | |||
| Aplysioidea (p. 430). | ||||
| Pleurobranchoidea (p. 431). | ||||
| Siphonarioidea (p. 431). | ||||
| Opisthobranchiata | Ascoglossa (p. 431). | |||
| Nudibranchiata | Cladohepatica (p. 432). | |||
| Holohepatica (p. 433). | ||||
| Pteropoda | Thecosomata (p. 435). | |||
| Gymnosomata (p. 437). | ||||
| Pulmonata | Basommatophora (p. 438). | |||
| Stylommatophora (p. 439). |
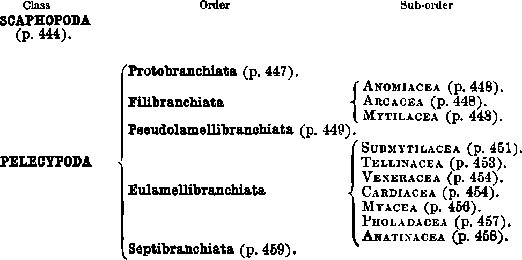
| Class | Order | Suborder |
| SCAPHOPODA (p. 444). | ||
| PELECYPODA | Protobranchiata (p. 447). | |
| Filibranchiata | Anomiacea (p. 448). | |
| Arcacea (p. 448). | ||
| Mytilacea (p. 448). | ||
| Pseudolamellibranchiata (p. 449). | ||
| Eulamellibranchiata | Submytilacea (p. 451). | |
| Tellinacea (p. 453). | ||
| Veneracea (p. 454). | ||
| Cardiacea (p. 454). | ||
| Myacea (p. 456). | ||
| Pholadacea (p. 457). | ||
| Anatinacea (p. 458). | ||
| Septibranchiata (p. 459). |
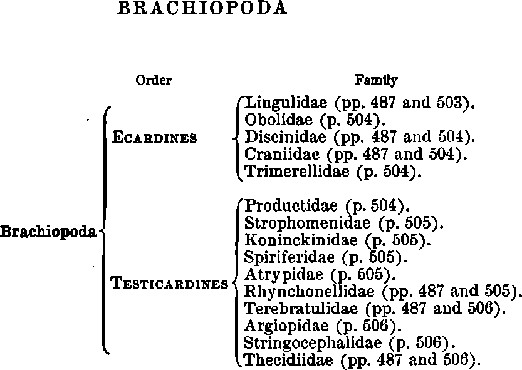
BRACHIOPODA
| Order | Family | |
| Brachiopoda | Ecardines | Lingulidae (pp. 487 and 503). |
| Obolidae (p. 504). | ||
| Discinidae (pp. 487 and 504). | ||
| Craniidae (pp. 487 and 504). | ||
| Trimerellidae (p. 504). | ||
| Testicardines | Productidae (p. 504). | |
| Strophomenidae (p. 505). | ||
| Koninckinidae (p. 505). | ||
| Spiriferidae (p. 505). | ||
| Atrypidae (p. 505). | ||
| Rhynchonellidae (pp. 487 and 505). | ||
| Terebratulidae (pp. 487 and 506). | ||
| Argiopidae (p. 506). | ||
| Stringocephalidae (p. 506). | ||
| Thecidiidae (pp. 487 and 506). |
[xi]
| The Geographical Distribution of the Land Operculate Mollusca | Frontispiece |
| The Geographical Distribution of the Land Mollusca of the East Indian Archipelago | Between pp. 308 and 309 |
| The Relations of the Land Mollusca of New Guinea with those of North Australia | To face p. 322 |
| The Geographical Distribution of the Land Mollusca of the West Indies | Between pp. 344 and 345 |
[xii]
BY
REV. A. H. COOKE, M.A.
Fellow and Tutor of King’s College, Cambridge
[1]
It is the generally accepted opinion among men of science that all life originated in the sea. Not that all parts of the sea are equally favourable to the development of forms of life. The ocean surface, with its entire absence of shelter or resting-place, and the deep sea, whose abysses are always dark and cold and changeless, offer little encouragement to plant or animal life, as an original starting-point. True, both the surface and the depths of the sea have become colonised by myriads of forms, Mollusca amongst them, but these quarters are in the truest sense colonised, for the ancestors of those who inhabit them in all probability migrated from elsewhere.
It was no doubt the littoral region and the shallow waters immediately below it, a region of changeable currents, of light and shade, of variation, within definite limits, of temperature and tide effects, which became the scene of the original development of plant life, in other words, of the food-supply which rendered possible its colonisation by higher animals. But the littoral region, besides the advantages of tenancy which it offers to animal life, has also its drawbacks. The violence of the surf may beat its inhabitants in pieces, the retreat of the tide exposes them, not merely to innumerable enemies in the shape of predatory birds and beasts, but also to a change in the atmospheric medium by which they are surrounded. Hence, in all probability, have arisen the various forms of adaptation which are calculated to bring about the ‘survival of the fittest’; hence, to[2] narrow our point of view to the Mollusca, the development of hard shells, or exoskeletons, hence the sand-burrowing, rock-boring, rock-clinging instincts of various genera and species.[1]
What was the primitive form of molluscan life is little likely to be ever positively known, although, on grounds of comparative anatomy, something approaching to the archi-mollusc is often constructed, with more or less probability, by careful observers. From one of the oldest known geological strata, the Cambrian, nearly four hundred species of Mollusca are known, which include representatives of nearly all the great Orders as they exist at the present day, and without the slightest sign of approximation to one another. With regard to the origin of the land and fresh-water Mollusca some definite conclusions can be arrived at, which will be given in their proper place.
Scarcely any portion of the coast-line of the world is destitute of molluscan life, except in regions where extreme cold forbids its existence. Thus along the shores of Northern Asia there is no proper littoral fauna, the constant influence of travelling ice sweeping it all away; animal life begins at about three fathoms. But in every coast region not positively hostile to existence Mollusca make their home. Each description of habitat has its own peculiar species, which there flourish best, and exist precariously, if at all, elsewhere. Thus the sandy waste of estuaries, the loose and shingly beaches, the slimy mud-flats beset with mangroves, the low stretches of jagged rock, and even the precipitous cliffs, from whose base the sea never recedes, have all their own special inhabitants. The same is true of the deep sea, and of the ocean surface. And when we come to examine the land and fresh-water Mollusca, it is found not merely that some Mollusca are terrestrial and others fluviatile, but that certain species haunt the hills and others the valleys, some the recesses of woods and others the open meadow sides, some prefer the limestone rocks, others the sandy or clayey districts, some live only in still or gently moving waters, while others are never found except where the current is rapid and powerful.
It is within the tropics that the Mollusca become most numerous, and assume their finest and quaintest forms. A tropical beach, especially where there is a good tide-fall and considerable[3] variety of station, abounds in molluscan life to an extent which must literally be seen to be believed. The beach at Panama, to select an instance familiar to the present writer, is astonishingly rich in species, which probably amount in all to several hundreds. This is due to the immense variety of habitat. On the rocks at high-water mark, and even above them, occur Truncatella, Melampus, Littorina, and Siphonaria; where a mangrove-swamp replaces the rock, on the branches overhead are huge Littorina, while three species of Cerithidea crawl on the mud, and Cyrena and Arca burrow into it. Lower down, in the rock pools at half-tide mark are Cerithium, Purpura, Omphalius, Anachis (2 sp.), Nassa, and several Crepidula. At low-water mark of ordinary tides, under stones half buried in clean sand, are Coecum and Vitrinella; under the blocks which rest on solid rock are Cypraea (4 or 5 sp.), Cantharus, more Anachis, Columbella (3 sp. including the graceful C. harpiformis), and Nitidella. Where the blocks of rock are rather muddy, Conus lurks, and with it Turritella and Latirus. Where the rocks form a flat-topped platform 2 or 3 feet high, with here and there a deep crack, huge Chitons 3 inches long conceal themselves, with two species of Turbo, Purpura, and Clavella. At extreme low-water mark of spring tides, on the isolated rocks are Monoceros, Leucozonia, and Vermetus, in them are Pholas and a burrowing Mytilus, under them are more Conus, Dolium, and huge frilled Murices. Patches of clean gravelly sand here produce Strombus; on the operculum of the great Str. galea is sure to be a Crepidula, exactly fitting its breadth. On the liquid mud-flats to the north glide about Marginella, Nassa, and Truncaria, in the clean sandstretch to the west Olivella ploughs about by hundreds with several species of Natica, and Tellina and Donax bury themselves deep, while farther down are Artemis, Chione, and, where mud begins to mix with the sand, Mytilus and more Arca. Each of these species has its own habitat, often circumscribed to a few square feet at the most, and it would be utterly useless to seek for it anywhere except in its own special domain.
Equally abundant are the land Mollusca of the tropics. Prof. C. B. Adams relates that within the limits of a single parish in Jamaica, named Manchester, which measures no more than four miles long and one mile broad, he obtained no fewer than one hundred species. Mr. J. S. Gibbons, in a description of[4] the Mollusca he obtained near St. Ann’s, Curaçao, gives a lively picture of their abundance in an exceptionally favoured locality:—[2]
“Near the outskirts of the town a waste piece of ground supplied me with occupation for all the time I had to spare. Neither grass nor water was to be seen, the only vegetation consisting of a few stunted cacti and still fewer acacia bushes. This, however, was so rich in shells that of several species enough specimens could have been collected in a few yards to supply, I should suppose, all the shell cabinets in the world.... The stones, plants, and ground were covered with Strophia uva L., Tudora megacheila, P. and M., was in equal abundance, suspended by its silk-like thread from acacia boughs, or strewed thickly on the ground underneath. A Bulimulus (B. multilineatus var. sisalensis) abounded on the smaller boughs, while under masses of coral Macroceramus inermis Gundl., Pupa parraiana d’Orb, and P. pellucida Pfr., were abundant. In the loose soil Cylindrella Raveni Bland, Cistula Raveni Bland, and a curious Cionella were so numerous that a spade would have been the best instrument with which to collect them. I wasted a good deal of valuable time in separating them from the soil, when by simply taking away a few handfuls of mould, I might have obtained a larger number of specimens. A species of Stenogyra and a Succinea complete a list, all of which might have been gathered from almost any square yard of ground on the hillside.”
Position of Mollusca in the Animal Kingdom.—Up to very recent times it was usual to regard the Mollusca as one of the four subdivisions of a great family known as Malacozoa, the subdivisions being (1) Mollusca, (2) Tunicata, (3) Brachiopoda, (4) Polyzoa or Bryozoa. This classification is still retained in the leading modern manual on the subject.[3] The progress, however, of investigation leads to the belief that the Mollusca are not so closely related to these other groups as such a classification would seem to imply. The Tunicata, for instance, appear, from the whole course of their development, to occupy[5] a position near to the Vertebrata. The relations of the Brachiopoda and Polyzoa will be more particularly referred to in that part of this History which deals especially with those groups. The position of the Mollusca is, in many respects, one of considerable isolation. Any attempt, therefore, definitely to relate them to one group or another, is, in all probability, to go further than the present state of our knowledge warrants. Especially to be deprecated are systems of classification which confidently derive the Mollusca in general from this or that group. The first undisputed traces of animal life, which appear in the Cambrian epoch, exhibit the same phyletic distinctions as now exist. Sponges, Echinoderms, Mollusca, and Worms, formed already, in those immeasurably remote ages, groups apparently as generally distinct from one another as they are at the present time. It would seem that any theory of development, which confidently teaches the derivation of any one of these groups from any other, is, in the present state of the evidence before us, hazardous in the extreme.
Some indications of relationship, which must not be pushed too far, may be drawn from a consideration of embryonic resemblance. An especial characteristic of the Mollusca is the possession of a particular form of larva, which occurs in one of the stages of development, known as the trochosphere (see p. 130). This form of larva is shared with two orders of Annelida, the Chaetopoda and the Gephyrea armata, and, in all probability, with the Polyzoa as well. It may also be significant that the adult form in Rotifera bears a close resemblance to the trochosphere larva in those groups.
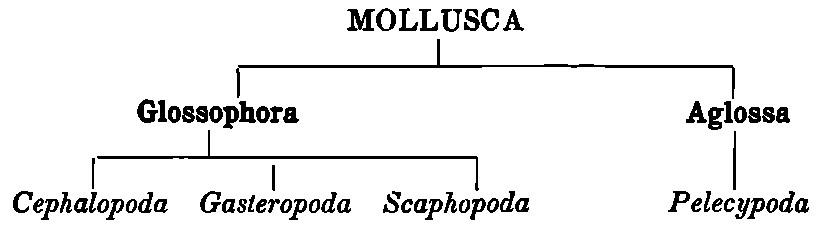
Basis of Classification.—The Mollusca are divided into four great Orders—Cephalopoda, Gasteropoda, Scaphopoda, and Pelecypoda.[4] Each name, it will be noticed, bears reference to the ‘foot,’ i.e. to the organ of motion which corresponds in function to the foot in the Vertebrata.
In the Cephalopoda the feet, or, as they are more frequently termed, the ‘arms,’ are arranged symmetrically round the head or mouth. The common forms of ‘cuttle-fish’ (Octopus, Loligo) are familiar examples of Cephalopods.
The Gasteropoda crawl on the flat under-surface or ‘sole’ of[6] the foot. Snails, slugs, sea-hares, whelks, periwinkles, and coats-of-mail or chitons are examples of this Order.
The Scaphopoda possess a long tubular shell open at both ends; with their small and elongated foot they are supposed to dig into the mud in which they live. The common Dentalium or tusk-shell of our coasts is a representative of this Order.
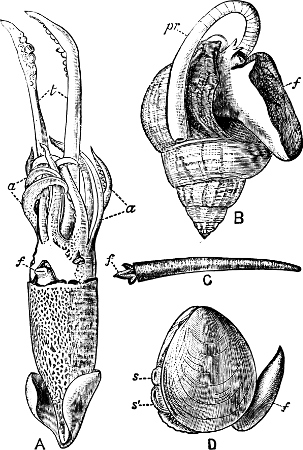
Fig. 1.—Examples of the four Orders: A, Cephalopoda; B, Gasteropoda; C, Scaphopoda, and D, Pelecypoda.
A, Ommastrephes sagittatus Lam., Naples: a, a, arms surrounding the mouth; f, funnel; t, t, the two ‘tentacular’ arms, × ⅖. B, Buccinum undatum L., Britain: f, foot; pr, proboscis. × ½. C, Dentalium entalis L., Norway: f, foot. D, Cardium oblongum Chem., Naples: f, foot; s, efferent or anal siphon; s’, efferent or branchial siphon, × ½.
The Pelecypoda[5] are enclosed in a bivalve shell fastened by a muscular hinge, the adjacent part of the valves being generally more or less toothed; the foot is as a rule roughly comparable to the shape of an axe-head.
To these four Orders is frequently added a fifth, the Pteropoda, whose exact position is at present not absolutely settled. The Pteropoda[6] are ‘pelagic,’ i.e. they live in the open waters of the ocean, rising to the surface at night, and sinking into cooler water by day. They are provided with a pair of wing-like[7] appendages or ‘feet,’ on each side of the head, by means of which they are enabled to swim. Some authorities regard the Pteropoda as a subdivision of Gasteropoda, others as forming a separate Order, of equivalent value to the other four. The question will be further discussed below (see chap. xv.), but for the present it will be sufficient to state that the weight of evidence appears to show that the Pteropoda are modified Gasteropoda, with special adaptations to pelagic life, and are therefore not entitled to rank as a separate Order.
Some writers conveniently group together the first three of these Orders, the Cephalopoda, Gasteropoda, and Scaphopoda, under the title Glossophora,[7] or Mollusca furnished with a radula or ribbon-shaped ‘tongue,’ set with rows of teeth and situated in something of the nature of a head, as distinguished from the Aglossa (or Lipocephala),[8] i.e. those Mollusca which have no radula and no head. To the latter belong only the fourth Order, the Pelecypoda. This view postulates, for the primitive ancestral Mollusc, a body with a more or less developed head, and possibly the rudiments of an apparatus for grinding or triturating food. This form, it is held, either developed or degenerated. In the former case, in consequence of the more active mode of life upon which it may be supposed to have entered, it gave rise to all the more highly organised forms which are grouped under the three great Orders. When, on the other hand, the ancestral form associated itself with an inactive or sedentary life, it was, we may believe, modified accordingly, and either lost by atrophy or failed to acquire those special points of organisation which characterise the highly-developed form. Hence the Pelecypoda, or bivalves, whose characteristic is the absence of any definite cephalic region or masticatory apparatus. It is a remarkable fact in support of this theory of the origin of the Aglossa that certain of their larvae are known to possess traces of higher organisation, e.g. an external mouth and eyes, the former of which becomes covered by the mantle lobes, while the latter disappear long before the adult stage is reached.
[8]
Thus we have
Classification of Gasteropoda.—The Gasteropoda are numerically very largely in excess of the two other Orders of the Glossophora, far more complicated as regards classification, and contain a large proportion of those examples of the Mollusca which are most familiar to the ordinary observer. It will therefore be convenient to postpone for the present a fuller discussion of the subdivisions of the Cephalopoda and Scaphopoda, as well as of the Aglossa, returning to them again in special chapters (chaps. xiii. and xvi.), and to devote a few introductory words to the classification and relations of the Gasteropoda.
The Gasteropoda are divided into four Classes, Amphineura, Prosobranchiata, Opisthobranchiata, and Pulmonata.
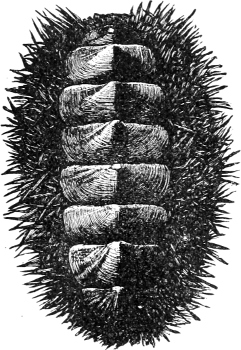
Fig. 2.—An example of the Polyplacophora: Chiton spinosus Brug.
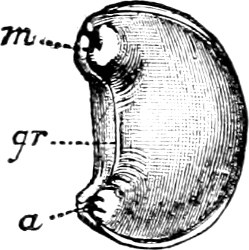
Fig. 3.—An example of the Aplacophora, Neomenia carinata Tullb.: a, anus; gr, ventral groove; m, mouth.
(1) The Amphineura[9] are bilaterally symmetrical Mollusca, i.e. with organs either single and central, or paired and disposed on either side of the longer axis of the animal. The shell, when[9] present, is never spiral, but consists of eight overlapping plates, kept together by an elliptical girdle. The Amphineura are divided into (a) Polyplacophora,[10] or Chitons, and (b) Aplacophora (Chaetoderma and Neomenia).
(2) The Prosobranchiata[11] are so named from the fact that the breathing organ (branchia or ctenidium[12]) is as a rule situated in front of the heart, the auricle at the same time being in front of the ventricle. They are asymmetrical, almost always furnished with a shell, which is at some time spiral, and with an operculum. The sexes are separate. They are either marine animals, or can be shown to be more or less directly derived from genera which are marine. They are divided into (a) Diotocardia[13] (Haliotis, Fissurella, Trochus, Nerita, Patella), which have, or whose immediate ancestors are believed to have had, two auricles to the heart, two sets of breathing organs, two kidneys, but no proboscis, penis, or siphon, and (b) Monotocardia,[14] in which the heart has only one auricle, the true breathing organ is single, and there is a single kidney. To this division belong the great majority of marine univalve Mollusca, e.g. Cypraea, Buccinum, Murex, Littorina, Ianthina, all the land and fresh-water operculates (Cyclostoma, Melania, Paludina, etc.), as well as the Heteropoda, which are a group of Prosobranchiata which have betaken themselves to a pelagic life.
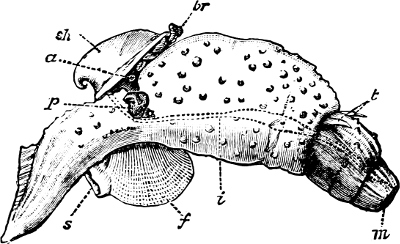
Fig. 4.—Example of a Heteropod, Carinaria mediterranea Lam., Naples: a, anus; br, branchia; f, foot; i, intestine; m, mouth; p, penis; s, sucker; sh, shell; t, tentacles. × ½. The animal swims foot uppermost.
(3) In the Opisthobranchiata[5] the breathing organs (when present) are behind the heart, and the auricle of the heart is consequently behind the ventricle. They are asymmetrical marine animals; usually, but by no means always, without a[10] shell, scarcely ever with an operculum in the adult state. The sexes are united in the same individual. The Opisthobranchiata fall into two divisions: (a) Tectibranchiata, in which the breathing organ is more or less covered by the mantle, and a shell is usually present, which is sometimes rudimentary, e.g. Bulla, Aplysia, Umbrella, and the whole group of Pteropoda; (b) Nudibranchiata, or sea slugs, which have no shell and no true ctenidia, but breathe either by the skin, or by ‘cerata’ or papilliform organs prominently developed on the back: e.g. Doris, Aeolis, Dendronotus.
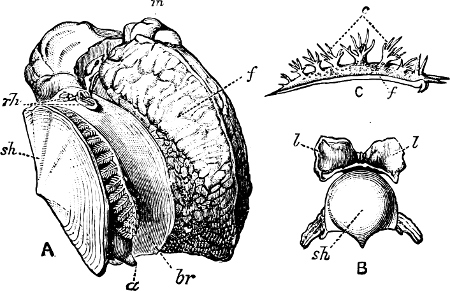
Fig. 5.—A, A Tectibranchiate Opisthobranch, Umbrella mediterranea Lam., Naples: a, anus; br, branchia; f, foot; m, mouth; rh, rhinophores; sh, shell.
B, A Pteropod, Hyalaea tridentata Forsk., Naples: sh, shell; l, l, swimming lobes of foot.
C, A Nudibranchiate Opisthobranch, Aeolis peregrina, Naples: f, foot; c, cerata.
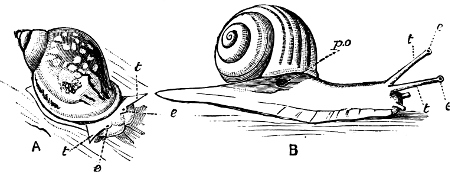
Fig. 6.—Examples of—A, Pulmonata Basommatophora, the common Limnaea peregra Müll.: e, e, eyes; t, t, tentacles. B, Pulmonata Stylommatophora, Helix hortensis Müll.: e, e, eyes; t, t, tentacles; p. o, pulmonary orifice (the position of the pulmonary orifice in Limnaea will be seen by reference to Fig. 101).
(4) The Pulmonata[15] are asymmetrical air-breathing non-marine Mollusca, generally, but not always, furnished with a shell. The sexes are always united in the same individual, and the operculum is always wanting, except in Amphibola. They[11] are conveniently divided into Stylommatophora,[16] in which the eyes are at the tip of the upper tentacles, which are retractile (Helix, Limax, Bulimus, and all true land slugs and snails), and Basommatophora, in which the eyes are at the base of the tentacles, which are not retractile (Limnaea, Planorbis, Physa, and all the Auriculidae).
Thus we have
| Gasteropoda | Amphineura | Polyplacophora |
| Aplacophora | ||
| Prosobranchiata | Diotocardia | |
| Monotocardia (incl. Heteropoda) | ||
| Opisthobranchiata | Tectibranchiata (incl. Pteropoda) | |
| Nudibranchiata[17] | ||
| Pulmonata | Stylommatophora | |
| Basommatophora |
The relation of the four great Orders to one another will be better discussed when we come to deal with each Order separately. The problem of the origin and mutual relationship of the various forms of molluscan life is of extreme subtlety, and its solution can only be approached after a comprehensive survey of many complicated anatomical details. But there is one branch of the Mollusca—the land and fresh-water genera—whose origin is, comparatively speaking, of recent date, and whose relationships are therefore less likely to have suffered complete obliteration.
Origin of the Land and Fresh-water Mollusca.—The ultimate derivation of the whole of the land and fresh-water molluscan fauna must, as has already been remarked, be looked for in the sea. In certain cases the process of conversion, if it may be so termed, from a marine to a non-marine genus, is still in progress, and can be definitely observed; in others the conversion is complete, but the modification of form has been so slight, or the date of its occurrence so recent, that the connexion is unmistakable, or at least highly probable; in others again, the modification has been so great, or the date of its occurrence so remote, that the actual line of derivation is obscured or at best only conjectural.
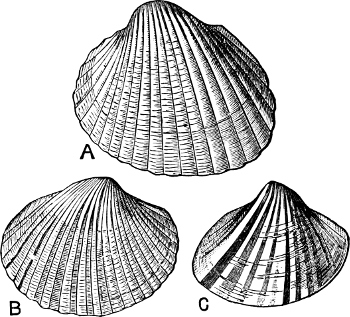
Fig. 7.—A, the common cockle (Cardium edule L.). B, Adacna plicata Eichw., Caspian Sea. C, Didacna trigonoides Pall., Caspian Sea.
This passage from a marine to a non-marine life—in other[12] words, this direct derivation of non-marine from marine genera—is illustrated by the faunal phenomena of an inland brackish-water sea like the Caspian, which is known to have been originally in connexion with the Mediterranean, and therefore originally supported a marine fauna. The Mollusca of the Caspian, although without exception brackish- or fresh-water species, are in their general facies distinctly marine. Of the 26 univalve species which inhabit it 19 belong to 4 peculiar genera (Micromelania, Caspia, Clessinia, Nematurella), all of which are modified forms of the marine Rissoidae. The characteristic bivalves belong to the genera Adacna, Didacna, and Monodacna, all of which can be shown to be derived from the common Cardium edule. We have here a case where complete isolation from the sea, combined no doubt with a gradual freshening of the water, has resulted in the development of a number of new genera. The singularly marine facies of several of the fresh-water genera now inhabiting Lake Tanganyika, has given rise to the belief, among some authorities, that that lake was at one time an inlet of the Indian Ocean. In the upper waters of the Baltic, marine and fresh-water Mollusca flourish side by side. So complete is the intermixture, that an observer who had lived on no other shores would probably be unable to separate the one set of species from the other.[18] Thus between Dagö and Papen-Wiek[19] Mytilus edulis, Cardium edule, Tellina balthica, Mya arenaria, Littorina rudis, and Hydrobia balthica are the only true marine species; with these live Unio, Cyclas, Neritina, Limnaea, and Bithynia. The marine species and Neritina live down to 15–20 fath., the[13] rest only down to 3 fath. Under stones close to the shore of the Skärgård at Stockholm[20] are found young Cardium and Tellina, and at 3 to 6 fath. Limnaea peregra, and Physa fontinalis. Near Gothland Limnaea is found in the open sea at 8–12 fath., and with it occur Cardium and Tellina. At the Frisches Haff[21] Mya arenaria is the only marine species, and lives in company with 6 sp. Limnaea, 1 Physa, 9 Planorbis, 1 Ancylus, 4 Valvata, 2 Sphaerium. Were the Sound to become closed, and the waters of the Baltic perfectly fresh, it would be inevitable that Mya arenaria, and such other marine species as continued to live under their changed conditions, should in course of time submit to modifications similar in kind to those experienced by the quondam marine species of the Caspian.
It seems probable, however, that the origin, at least in a great part, of the land and fresh-water Mollusca need not be accounted for by such involuntary changes of environment as the enclosure of arms of the sea, or the possible drying up of inland lakes. These cases may be taken as illustrations of the much more gradual processes of nature by which the land and fresh-water fauna must have been developed. The ancestry of that fauna must be looked for, as far as the Gasteropoda are concerned, in the littoral and estuarine species; for the Pelecypoda, in the estuarine alone. The effect of the recess of the tide, in the one case, and the effect of the reduced percentage of salt, in the other, has tended to produce a gradual adaptation to new surroundings, an adaptation which becomes more and more perfect. It may be safely asserted that no marine species could pass into a land or fresh-water species except after a period, more or less prolonged, of littoral or estuarine existence. Thus we find no land or fresh-water species exhibiting relationships with such deep-sea genera as the Volutidae, Cancellariidae, Terebridae, or even with genera trenching on the lowest part of the littoral zone, such as the Haliotidae, Conidae, Olividae, Capulidae. The signs of connexion are rather with the Neritidae, Cerithiidae, and above all the Littorinidae, which are accustomed to live for hours, and in the case of Littorina for days or even weeks, without being moistened by the tide. Similarly the[14] fresh-water Pelecypoda exhibit relationships, not with genera exclusively marine, but with genera known to inhabit estuaries, such as the Mytilidae, Corbulidae, Cardiidae.
It would be natural to expect that we should find this process of conversion still going on, and that we should be able to detect particular species or groups of species in process of emigration from sea to land, or from sea to fresh water. Such species will be intermediate between a marine and a land or fresh-water species, and difficult to classify distinctly as one or the other. Cases of Mollusca occupying this intermediate position occur all over the world. They inhabit brackish swamps, damp places at high-water mark, and rocks only at intervals visited by the tide. Such are Potamides, Assiminea, Siphonaria, Melampus, Hydrobia, Truncatella, among the univalves, and many species of Cyrena and Arca among the bivalves.
(a) Pelecypoda.—Estuarine species, which have become accustomed to a certain admixture of fresh water, have gradually ascended the streams or been cut off from the sea, and have at last become habituated to water which is perfectly fresh.
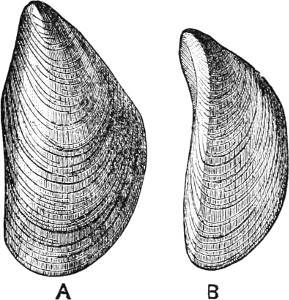
Fig. 8.—A, The common Mytilus edulis L., a marine genus and species. B, Dreissensia, a fresh-water genus, closely allied to Mytilus.
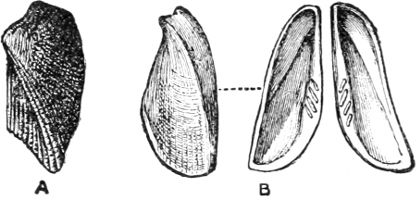
Fig. 9.—A, Arca navicella Reeve, Philippines, a marine species. B, Arca (Scaphula) pinna Bens., R. Tenasserim, a fresh-water species which lives many miles above the tide-way.
Thus Dreissensia (rivers and canals throughout N. Europe and N. America) and Mytilopsis (rivers of America) are scarcely modified Mytili (Fig. 8); Scaphula is a modified Arca,[15] and lives in the Ganges, the Jumna, and the Tenasserim at a distance of 1600 miles from the sea (Fig. 9). Pholas rivicola is found imbedded in floating wood on the R. Pantai many miles from its mouth. Cyrena, Corbicula, and probably Sphaerium and Pisidium are derived, in different degrees of removal, from the exclusively marine Veneridae; Potamomya (rivers of S. America), and Himella (R. Amazon) are forms of Corbula. The Caspian genera derived from Cardium (Adacna, Didacna, Monodacna), have already been referred to. Nausitora is a form of Teredo, which lives in fresh water in Bengal. Rangia, Fischeria, and Galatea probably share the derivation of the Cyrenidae, while in Iphigenia we have one of the Donacidae which has not yet mounted rivers, but is confined to a strictly estuarine life. The familiar Scrobicularia piperata of our own estuaries is a Tellina, which lives by preference in brackish water.
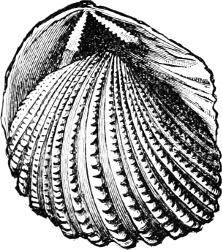
Fig. 10.—Trigonia pectinata Lam., Sydney, N.S.W.
The great family of the Unionidae is regarded by Neumayr[22] as derived from Trigonia, the points of similarity being the development of a nacreous shell, the presence of a strong epidermis, and the arrangement of the muscular scars. It is remarkable, too, that on many Uniones of Pliocene times there is found shell ornamentation of such a type as occurs elsewhere among the Pelecypoda only on Trigonia.
The genera of fresh-water Pelecypoda are comparatively few in number, and their origin is far more clearly discernible than that of any other group. This is perhaps due to the fact that the essential changes of structure required to convert a marine into a fresh-water bivalve are but slight. Both animals “breathe water,” and both obtain their nutriment from matter contained in water. Similar remarks apply to fresh-water operculate Gasteropoda. But the passage from a marine to an aerial life involves much profounder changes of environment, which have to be met by correspondingly important changes in the organism. This may be in part the[16] reason why the ancestry of all Pulmonata, whether land or fresh-water, is so difficult to trace.
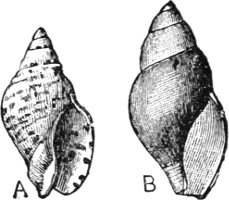
Fig. 11.—A, Cominella, a marine genus, which lives between tide marks, and from which is probably derived B, Clea, a genus occurring only in fresh water.
Fig. 12.—A, Cerithium columna Sowb. (marine). B, Potamides microptera Kien. (brackish water). C, Io spinosa Lea, one of the Pleuroceridae (fresh water).
(b) Gasteropoda.—(1) Operculate. Canidia and Clea are closely allied, with but little modification, to the marine Cominella[23] (Fig. 11), as is also Nassodonta to Nassa. They occur (in fresh water) in the rivers of India, Indo-China, Java, and Borneo, associated with essentially fresh-water species. Potamides, with its various sub-genera (Telescopium, Pyrazus, Pirenella, Cerithidea, etc.), all of which inhabit swamps and mud-flats just above high-water mark in all warm countries, are derived from Cerithium (Fig. 12); Assiminea, Hydrobia, and perhaps Truncatella, from Rissoa. It is a remarkable fact that in Geomelania (with its sub-genera Chittya and Blandiella) we have a form of Truncatella which has entirely deserted the neighbourhood of the sea, and lives in woody mountainous localities in certain of the West Indies. Cremnoconchus, a remarkable shell occurring only on wet cliffs in the ghâts of southern India, is a modified Littorina. Neritina and Nerita form a very interesting case in illustration of the whole process. Nerita is a purely marine genus, occurring on rocks in the littoral zone; one species, however, (N. lineata,[17] Chem.) ascends rivers as far as 25 miles from their mouth, and others haunt marshes of brackish water. Neritina is the fresh-water form, some species of which are found in brackish swamps or even creeping on wet mud between tide marks, while the great majority are fluviatile, one group (Neritodryas) actually occurring in the Philippines on trees of some height, at a distance of a quarter of a mile from any water. Navicella is a still further modified form of Neritina, occurring only on wet rocks, branches, etc., in non-tidal streams (Fig. 13).
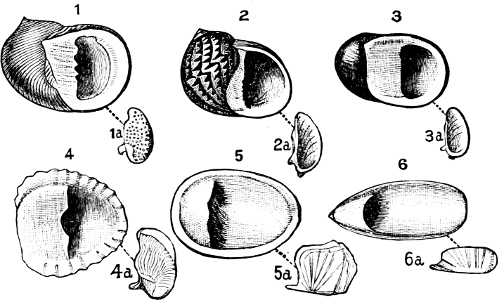
Fig. 13.—Illustrating the development of the fresh-water genus Navicella, through the brackish-water Neritina, from the marine Nerita, with corresponding changes in the operculum. 1. Nerita; 2, 3. Neritina; 4. Neritina, intermediate form; 5, 6. Navicella.
The great family of the Melaniidae, which occurs in the rivers of warm countries all over the world, and that of the Pleuroceridae, which is confined to North America, are, in all probability, derived from some form or forms of Cerithium. The origin of the Paludinidae, Valvatidae, and Ampullariidae is more doubtful. Their migration from the sea was probably of an early date, since the first traces of all three appear in the lower Cretaceous, while Melaniidae are not known until Tertiary times. Ampullaria, however, shows distinct signs of relationship to Natica, while the affinities of Paludina and Valvata cannot as yet be approximately affirmed.
(2) Pulmonata.—Intermediate between the essentially fresh-water and the essentially marine species come the group sometimes known as Gehydrophila, consisting of the two families Auriculidae and Otinidae. These may be regarded as Mollusca which, though definitely removed from all marine species by the development of a true lung or lung cavity in the place of a gill,[18] have yet never become, in respect of habitat, genuine fresh-water species. Like Potamides, they haunt salt marshes, mangrove swamps, and the region about high-water mark. In some cases (Otina, Melampus, Pedipes) they live on rocks which are moistened, or even bathed by the spray, in others (Cassidula, Auricula) they are immersed in some depth of brackish water at high tide, in others again (Scarabus) they are more definitely terrestrial, and live under dead leaves in woods at some little distance from water. Indeed one genus of diminutive size (Carychium) has completely abandoned the neighbourhood of the sea, and inhabits swampy ground almost all over the world.
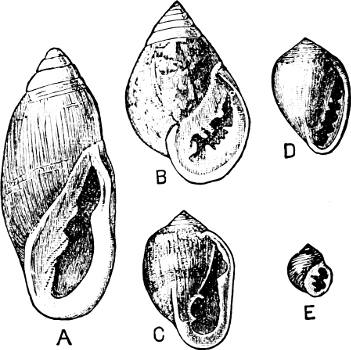
Fig. 14.—Examples of the Auriculidae: A, Auricula Judae Lam., Borneo; B, Scarabus Lessoni Blainv., E. Indies; C, Cassidula mustelina Desh., N. Zealand; D, Melampus castaneus Mühlf., S. Pacific; E, Pedipes quadridens Pfr., Jamaica.
Fig. 15.—An example of Amphibola (avellana Chem.), the only true Pulmonate which possesses an operculum.
To this same section Gehydrophila have been assigned two remarkable forms of air-breathing “limpet,” Siphonaria and Gadinia (see page 151), and the aberrant Amphibola, a unique instance of a true operculated pulmonate. Siphonaria possesses a pulmonary cavity as well as a gill, while Gadinia and Amphibola are exclusively air-breathing. Siphonaria lives on rocks at or above high-water mark, Gadinia between tide marks, Amphibola (Fig. 15) in brackish water at the estuaries of rivers, half buried in the sand. There can be little doubt that all these are marine forms which are gradually becoming accustomed to a terrestrial existence. In Gadinia and Amphibola the process is so far complete that they have exchanged gills for a pulmonary cavity, while in Siphonaria we have an intermediate stage in which both organs exist together. A curious parallel to this is found in the case of Ampullaria, which is furnished with two gills and a pulmonary chamber, and breathes indifferently air and water. It is a little[19] remarkable that Siphonaria, which lives at a higher tide level than Gadinia, should retain the gill, while Gadinia has lost it.
The ultimate affinities of the essentially fresh-water groups, Limnaea, Physa, Chilina, cannot be precisely affirmed. The form of shell in Latia, Gundlachia, and perhaps Ancylus, may suggest to some a connexion with the Otinidae, and in Chilina, a similar connexion with the Auriculidae. But, in a question of derivation, similarities of shell alone are of little value. It is not a little remarkable, for instance, that we should find a simple patelliform shell in genera so completely distinct from one another in all anatomical essentials as Ancylus, Patella, Siphonaria, Propilidium, Hipponyx, Cocculina, and Umbrella.
Some recent authors, on grounds of general organisation, regard the Limnaeidae and their allies as Opisthobranchs adapted to an aerial life. It is held[24] that the Nudibranchiate Opisthobranchs have given birth to the Pulmonata Stylommatophora or land snails, and the Tectibranchiate Opisthobranchs to the Pulmonata Basommatophora or fresh-water snails. Such a view seems at first sight open to some objection from other views than those which deal simply with anatomy. The Opisthobranchiata are not, to any marked extent, littoral genera, nor do they specially haunt the mouths of rivers. On the contrary, they inhabit, as a rule, only the very lowest part of the littoral zone, and are seldom found, except where the water is purely salt. In other cases, when the derivation of land or fresh-water genera is fairly well established, intermediate forms persist, which indicate, with more or less clearness, the lines along which modification has proceeded. It has, however, recently been shown that Siphonaria[25] and Gadinia,[26] which have, as has been already mentioned, hitherto been classified as Pulmonata, are in reality modified forms of Opisthobranchiata, which are in process of adaptation to a life partly marine, partly on land. They may therefore be regarded as supplying the link, hitherto missing, between the land Pulmonata and the marine groups from one or other of which the latter must have been derived. The general consensus of recent opinion inclines towards accepting these views, some writers[27] being content to regard the[20] Pulmonata, as a whole, as derived from the Tectibranchiate Opisthobranchs, while others[28] go further and regard the Stylommatophora as derived directly from the Basommatophora.
Gasteropoda.—(1) Operculate. On a priori grounds, one might predict a double origin for land operculates. Marine species might be imagined to accustom themselves to a terrestrial existence, after a period, more or less prolonged, of littoral probation. Or again, fresh-water species, themselves ultimately derived from the sea, might submit to a similar transformation, after a preliminary or intermediate stage of life on mudbanks, wet swamps, branches overhanging the water, etc. Two great families in this group, and two only, seem to have undergone these transformations, the Littorinidae and the Neritidae. The derivation of almost all existing land operculates may be referred to one or other of these groups.
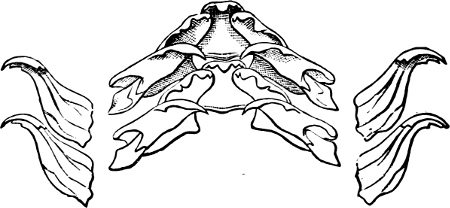
Fig. 16.—Two rows of the radula of Littorina littorea L., × 72.
The power of the Littorinidae to live for days or even weeks without being moistened by the sea may be verified by the most casual observer. In the tropics this power seems even greater than on our own shores. I have seen, in various parts of Jamaica, Littorina muricata living at the top of low cliffs among grass and herbage. At Panama I have taken three large species of Littorina (varia, fasciata, pulchra), on trees at and above high-water mark. Cases have been recorded in which a number of L. muricata, collected and put aside, have lived for three months, and L. irrorata for four months.[29] These facts are significant, when we know that the land operculates almost certainly originated in a tropical climate.
[21]
The Cyclophoridae, Cyclostomatidae, and Aciculidae, which, as contrasted with the other land operculates, form one group, have very close relations, particularly in the length and formation of the radula, or lingual ribbon, with the Littorinidae.
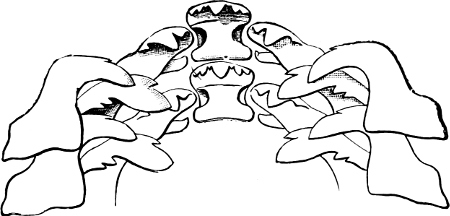
Fig. 17.—Two rows of the radula of Cyclophorus sp., India, × 40.
On the other hand, the Helicinidae, Hydrocenidae, and Proserpinidae are equally closely related to Neritina. The Proserpinidae (restricted to the Greater Antilles, Central America and Venezuela) may perhaps be regarded as the ultimate term of the series. They have lost the characteristic operculum, which in their case is replaced by a number of folds or lamellae in the interior of the shell. It has already been noticed how one group of Neritina (Neritodryas) occurs normally out of the water. This group furnishes a link between the fresh-water and land forms. It is interesting to notice that here we have the most perfect sequence of derivatives; Nerita in the main a purely marine form, with certain species occurring also in brackish water; Neritina in the main fresh-water, but some species occurring on the muddy shore, others on dry land; Helicina the developed land form; and finally Proserpina, an aberrant derivative which has lost the operculum.[30]
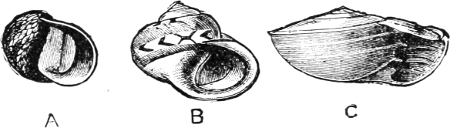
Fig. 18.—A, Neritina reticularis Sowb., Calcutta (brackish water); B, Helicina neritella Lam., Jamaica (land); C, Proserpina (Ceres) eolina Ducl., Central America (land).
[22]
Gasteropoda.—(2) Pulmonata. The origin of these, the bulk of the land fauna, must at present be regarded as a problem not yet finally solved. Some authorities, as we have seen, regard them as derived from the Nudibranchiate, others, probably more correctly, from the Tectibranchiate Opisthobranchs.
The first known members of the land Pulmonata (Pupa [?], Hyalinia) are from the Carboniferous of North America. Similar but new forms appear in the Cretaceous, from which time to the present we have an unbroken series. The characteristically modern forms, according to Simroth,[31] are Helices with thick shells. According to the same author, Vitrina and Hyalinia are ancestral types, which give origin not only to many modern genera with shells, but to many shell-less genera also, e.g. Testacella is probably derived through Daudebardia from Hyalinia, while from Vitrina came Limax and Amalia. A consideration of the radulae of the genera concerned certainly tends in favour of these views.
Godwin-Austen, speaking generally, considers[32] genera of land Pulmonata with strongly developed mantle-lobes and rudimentary shell as more advanced in development than genera in which the shell is large and covers all or nearly all the animal.
[23]
The majority of the Land Mollusca are probably more sensitive than is usually believed. The humidity of the air must affect the surface of their skin to a considerable extent. Every one has noticed how the snails ‘come out’ on a damp evening, especially after rain. As a rule, they wait till rain is over, probably objecting to the patter of the drops upon their delicate tentacles. Snails kept in captivity under a bell-glass are acutely sensitive of a damp atmosphere, and will bestir themselves after rain just as if they were in the open air. Certain Helices which are accustomed to live in moist places, will find their way to water, if removed from their usual haunts. A case is recorded[33] of a specimen of H. arbustorum, kept in a kitchen, which used to find its way directly under the cold water tap, and appeared to enjoy the luxury of a douche. How delicately the conditions of life are balanced in some of these creatures is seen in the case of Omalonyx, a genus akin to Succinea, which is found in Brazil and the northern parts of South America. It lives creeping on plants which overhang the margin of water, but perishes equally, if placed in the water itself, or removed to a distance from it for any length of time.[34]
Endurance of Heat and Cold.—The Mollusca are capable, at least as far as some species are concerned, of enduring severe extremes both of cold and heat. The most northern pulmonate yet observed is a fresh-water species, Physa (Aplecta) hypnorum L. This hardy mollusc, whose shell is so fragile as to need most careful handling, has been noticed on the peninsula of Taimyr, North Siberia, in 73° 30’ N. lat, a region whose mean annual[24] temperature is below 10° F. with a range of from 40° F. in July to -30° F. in January.
It is well known that the Limnaeidae, and probably most fresh-water Mollusca of sub-temperate regions, can continue to live not merely under, but enveloped in ice, and themselves frozen hard. Garnier relates[35] that, during the winter of 1829–30, some large Limnaea auricularia, which had been placed in a small basin, were frozen into a solid mass, experiencing a cold of -2° F. He supposed they were dead, but, to his surprise, when the basin thawed, the Limnaea gradually revived. Paludina vivipara and Anodonta anatina have been known to resist a temperature of 23° F., and the former has produced young shortly after being thawed out of the ice.[36] As far north as Bodø in Norway (67° 37’ N. lat., well within the Arctic circle) there are found no less than fourteen species of terrestrial Mollusca, among them being Balea perversa and Clausilia rugosa.[37]
Vitrina is one of our most hardy molluscs, and may be observed crawling on bright mornings over the frost-covered leaves of a wood or copse. V. glacialis is said by Charpentier to live in the Alps at a height where the stones are covered with snow from nine to ten months of the year. Many of the Hyaliniae are very hardy. Arion, in spite of having no external shell to protect it, is apparently less affected by the cold than Helix, and does not commence hibernation till a later period in the autumn. The operculate land Mollusca, in spite of the protection which their operculum may be supposed to afford, are exceedingly sensitive to cold, and the whole group is without doubt a product of tropical or semi-tropical regions (see map at frontispiece). A species of Helicina which inhabits the southern States of North America has been known to be almost exterminated from certain districts by the occurrence of an unusually severe winter.
One of the highest altitudes at which a land shell is known to live appears to be the Liti Pass (Himalayas, 14,000 ft.). At this enormous altitude, two species of Buliminus (arcuatus Hutt. and nivicola Bens.) live on juniper bushes among patches of snow. An Anadenus is said to have been found in a similar[25] locality at 15,000 ft., while Limnaea Hookeri has been taken from over 16,400 ft. in Landour. In the Andes of Peru and Bolivia, five species of Bulimulus, one of Pupa, and one of Limax occur at an elevation of 10,500 to 15,000 ft. Several fresh-water Mollusca inhabit Lake Titicaca, which stands at a height of 12,550 ft. in the Bolivian table-land.
In certain parts of the desert of Algeria, where there is not a trace of vegetation to be seen, and the temperature at mid-day is 110° F., the ground is sometimes so covered with Helix lactea as to appear perfectly white. Dr. F. H. H. Guillemard has told me that he noticed, in somewhat similar surroundings between Fez and Tangier, H. pisana in such extraordinary abundance that they hung from the low scrub in bunches the size of a man’s two fists. It is singular that Mollusca should live, and not only live, but flourish, in localities apparently so unpromising. Shells which occur in the Algerian Sahara are actually larger and altogether finer than the ordinary European form of the same species. In order to protect themselves to some extent against the scorching heat and consequent evaporation, desert species are frequently modified in one of two ways; the shell becomes either white or a light dusky brown, as in the familiar Helix desertorum, or else it gains immensely in thickness. Specimens of H. pomatia, recently procured from Fez, are of extraordinary thickness as compared with forms from our own chalk downs of Kent and Surrey.
Fresh-water Mollusca are frequently found inhabiting hot springs. Thus Neritina fluviatilis lives at Bagnères de Bigorre in water at about 68° F. In another hot spring in the eastern Pyrenees a Bithynia lives at a temperature of over 73° F.; while Blainville mentions another case of a Bithynia living in water at 122° F.
Hibernation and Aestivation.—As autumn begins to draw on, and the first frosts to nip vegetation, terrestrial species retire beneath stones, into cracks in old walls, holes in tree trunks, deep fissures in rocks, and nooks and crannies of every kind, or else bury themselves deeply in the earth or in moss and heaps of leaves. They thus commence their period of hibernation, which varies in length according to the duration of winter. Frequently masses of Helices may be found attached to one another, probably not so much for the sake of warmth, for their[26] temperature is but low, as to share the comforts of a cosy retreat in common. Slugs generally hibernate alone, excavating a sort of nest in the earth, in which they encyst themselves, contracting their bodies until they are almost round, and secreting a covering of their own slime. The Helices usually close up the mouth of their shell by the formation of a membranous or chalky epiphragm, which will be further described below. Both snails and slugs take care to be in good condition at the time their winter sleep begins, and for this reason the former are said to be most esteemed by foreign epicures if captured just at this period.[38]
During hibernation, the action of the heart in land Pulmonata ceases almost entirely. This appears to be directly due to the effect of cold. Mr. C. Ashford has related[39] some interesting experiments made upon H. hortensis and Hyal. cellaria, with the view of ascertaining the effect of cold upon their pulsations. His observations may be tabulated as follows:—
| Number of pulsations per minute | ||
| Helix hortensis | Hyal. cellaria | At degrees Fahr. |
| 22 | 21 | 52° |
| 14 | 12 | 44° |
| 10 | 11 | 38° |
| 4 | 9 | 30° |
At low temperatures the character, as well as the number of the pulsations changed; they became imperfect and intermittent, although exceptionally at 31° F. a H. rufescens gave five or six pulsations a minute, very full and deliberate. The result of taking the Hyalinia suddenly into the heat of a greenhouse was to bring on palpitations. Further experiments resulted in evidence of a similar kind. Hyal. radiatula, placed upon a deal table in a room, showed 52 pulsations per minute at 62° F. Placed upon the palm of the hand, the action soon rose to 108. Hyal. alliaria, similarly treated, rose from 72 pulsations to 110. Floated upon water, the action of the heart of the latter suddenly fell to 29.
Fresh-water Pulmonata do not appear to hibernate. Unio and Anodonta, however, bury themselves more deeply in the mud, and Dreissensia casts off its byssus and retires under the[27] mud in deeper water.[40] Limnaea and Planorbis have often been noticed to crawl about under the lower surface of a thick coating of ice. In periods of prolonged drought, when the water in the ponds dries up, the majority of genera bury themselves in the mud. I have known Limnaea peregra bury itself three inches deep, when surprised by a sudden fall of the water in the ditch on Coe Fen, behind Peterhouse, Cambridge. Physa hypnorum frequents by preference ditches which dry up in summer, as does also Planorbis spirorbis, the latter often forming a sort of epiphragm against evaporation. Ancylus has been observed to spend the whole winter out of water, and P. spirorbis has been noticed alive after four months’ desiccation.[41]
True aestivation, however, occurs mainly in the tropics, where there is no winter, but only a period when it is not quite so hot as the rest of the year, or on a coast like the Mediterranean, which is subject to sudden and severe heat. This period is usually rainless, and the heat is therefore a dry heat. At this season, which may last for three or four months, most of the land Mollusca enter upon a period of inaction, either burying themselves deeply in the ground, or else permanently attaching themselves to the stalks of grass and other herbage, or the under sides of rocks. For instance, the large and beautifully painted Orthalicus, Corona, and Porphyrobaphe, which inhabit Brazil, Ecuador, and eastern Peru, bury themselves deeply in the ground during the dry season, while in the rains they climb to the topmost branches of the great forest trees.[42] Thus it may well happen that a visitor to a tropical island, Ceylon for instance, or one of the Greater Antilles, if he times his visit to coincide with the rainless season, may be grievously disappointed at what seems its unaccountable poverty in land Mollusca. But as soon as the weather breaks, and the moisture penetrates their retreats, every bush and every stone, in favoured localities, will be alive with interesting species.
The Epiphragm.—A considerable number of the land Pulmonata (and a very few of the fresh-water) possess the power of closing the aperture of their shell by means of what is known as an epiphragm or covering of hardened mucus. This epiphragm is habitually formed by certain species during hibernation[28] or aestivation, or even during shorter periods of inactivity and retirement, the object being, either to check evaporation of the moisture of the body, or to secure the animal against the cold by retaining a thin layer of slightly warm air immediately within the aperture of the shell.
The epiphragm differs widely in character in different species, sometimes (Clausilia, Pupa, Planorbis) consisting of the merest pellicle of transparent membrane, while at others (Helix aperta, H. pomatia) it is a thick chalky substance, with a considerable admixture of carbonate of lime, with the consistency of a hardened layer of plaster of Paris. Within these extremes every variety of thickness, solidity, and transparency occurs. During long hibernation several epiphragms are not unfrequently formed by the same individual snail, one within the other, at gradually lessening distances. The epiphragm thus performs, to a certain extent, the part of an operculum, but it must be remembered that it differs radically from an operculum physiologically, in being only a temporary secretion, while the operculum is actually a living part of the animal.
The actual mode of formation of the epiphragm would seem to differ in different species. According to Fischer,[43] the mollusc withdraws into its shell, completely blocking all passage of air into the interior, and closing the pulmonary orifice. Then, from the middle part of the foot, which is held exactly at the same plane as the aperture, is slowly secreted a transparent pellicle, which gradually thickens, and in certain species becomes calcareous. Dr. Binney, who kept a large number of Helix hortensis in confinement, had frequently an opportunity of noticing the manner in which the epiphragm was formed.[44] The aperture of the shell being upward, and the collar of the animal having been brought to a level with it, a quantity of gelatinous matter is thrown out [? where from]. The pulmonary orifice is then opened, and a portion of the air within suddenly ejected, with such force as to separate the viscid matter from the collar, and to project it, like a bubble of air, from the aperture. The animal then quickly withdraws farther into the shell, and the pressure of the external air forces back the vesicle to a level with the aperture, when it hardens and forms[29] the epiphragm. In some of the European species in which the gelatinous secretion contains more carbonate of lime, solidification seems to take place at the moment when the air is expelled, and the epiphragm in these is in consequence strongly convex.
Thread-spinning.—A considerable number of fresh-water Mollusca possess the power of stretching a thread, which is no more than an exceedingly elongated piece of mucus, to the surface of the water, and of using it as a means of locomotion. This thread bears no analogy whatever to the fibrous byssus of certain bivalves, being formed in an entirely different manner, without the need of a special gland.
The threads are ‘spun’ by several species of Limnaea, Physa, and Planorbis, by Bithynia tentaculata, and several of the Cycladidae. They are anchored to the surface by a minute concavity at the upper end, which appears to act like a small boat in keeping the thread steady. The longest threads are those of the Physae, which have been noticed to attain a length, in confinement, of 14 inches. They are always spun in the ascent, and as a rule, when the animal descends, it rolls the thread up and carries it down as it goes. A single thread is never spun on the descent, but occasionally, when a thread has become more or less of a permanence, it becomes stronger by the addition of more mucus each time it is used, whether for ascending or descending purposes. Cyclas cornea appears to be an exception to the rule that threads are only spun on the ascent. This species, which is particularly fond of crawling along the under surface of the water, has been noticed to spin a thread half an inch in length while on the surface, and to hang suspended from it for a considerable time.
What the exact use of the thread may be, must to a certain extent be matter of conjecture. The Limnaeidae are, in the great majority of cases, compelled to make periodic visits to the surface in order to inspire oxygen. It is also a favourite habit with them to float just under the surface, or crawl about on its under side, perhaps in pursuit of tiny vegetable organisms. Whatever may be the object of an excursion to the surface, a taut thread will obviously be a nearer way up than any other which is likely to present itself; indeed, without this thread-spinning power, which insures a tolerably rapid arrival at the[30] surface, the animal might find itself asphyxiated, or at least seriously inconvenienced, before it could succeed in taking in the desired supply of oxygen. With the Cycladidae, which do not breathe air, such an explanation is out of place; in their case the thread seems to be a convenient means of resting in one position in the intervals of the periods of active exercise to which several of the species are so much addicted.
The power of suspension by a thread is also possessed by certain of the Cyclostomatidae, by some Cerithidea, several Rissoa and other marine genera, prominent among which is Litiopa bombyx, whose name expresses its power of anchoring itself to the Sargasso weed by a silken thread of mucus. Several species of slugs are known to be able to let themselves down by threads from the branches of trees. Limax arborum is especially noted for this property, and has been observed suspended in pairs during the breeding time. According to Binney, all the American species of Limax, besides those of Tebennophorus, possess this singular property. Limax arborum appears to be the only slug which has been noticed to ascend, as well as descend, its thread. It has also been observed[45] that when this species is gorged with food, its slime is thin and watery, and unable to sustain its weight, but that after the process of digestion has been performed, the mucus again becomes thick and tenacious. It appears therefore that when the animal is hungry and most in need of the power of making distant excursions in search of food, its condition enables it to do so, but that when no such necessity is pressing, the thread-forming mucus is not secreted, or is perhaps held in suspense while the glands assist in lubricating the food before digestion.[46]
Food of Land and Fresh-water Mollusca.—Arion ater, the great black slug, although normally frugivorous, is unquestionably carnivorous as well, feeding on all sorts of animal matter, whether decaying, freshly killed, or even in a living state. It is frequently noticed feeding on earthworms; kept in captivity, it will eat raw beef; it does not disdain the carcases of its own dead brethren. An old man near Berwick-on-Tweed, going out one morning to mow grass, found a black slug devouring, as he supposed, a dead mouse. Being of an inquisitive[31] turn, and wishing to ascertain if it were really thus engaged, he drew the mouse a little back. When he returned in the evening, the mouse was reduced almost to a skeleton, and the slug was still there.[47] Indeed it would seem almost difficult to name anything which Arion ater will not eat. Dr. Gray mentions[48] a case of a specimen which devoured sand recently taken from the beach, which contained just enough animal matter to render it luminous when trodden on in the dark; after a little time the faeces of the slug were composed of pure sand, united together by a little mucus. A specimen kept two days in captivity was turned out on a newspaper, and commenced at once to devour it. The same specimen ate dead bodies of five other species of slugs, a dead Unio, pupae of Adimonia tanaceti, part of the abdomen of a dragon-fly, and Pears’ soap, the latter reluctantly.[49]
According to Simroth[50] and Scharff[51] the food of several of our British slugs, e.g. Limax maximus, L. flavus, Arion subfuscus, A. intermedius, consists of non-chlorophyllaceous substances only, while anything containing chlorophyll is as a rule refused. On the other hand L. agrestis and Amalia carinata feed almost entirely on green food, and are most destructive in gardens. The latter species lives several inches under ground during the day, and comes to the surface only at night. It is largely responsible for the disappearance of bulbs, to which it is extremely partial. L. marginatus (= arborum Bouch.) feeds exclusively on lichens, and in captivity absolutely refuses green leaves and a flesh diet. It follows therefore, if these observations are correct, that the popular notions about slugs must be revised, and that while we continue to exterminate from our gardens those species which have a taste for chlorophyll, we ought to spare, if not encourage those whose tastes lie in the opposite direction.
Limax agrestis has been seen devouring the crushed remains of Arion ater. Five specimens of the same species were once noticed busily devouring a May-fly each, and this in the middle of a large meadow, where it may be presumed there was no lack[32] of green food. The capture and eating of insects by Mollusca seems very remarkable, but this story does not stand alone. Mr. T. Vernon Wollaston once enclosed in a bottle at least three dozen specimens of Coleoptera together with 4 Helix cantiana, 5 H. hispida, and 1 H. virgata, together with an abundant supply of fresh leaves and grass. About a fortnight afterwards, on the bottle being opened, it was found that every single specimen of the Coleoptera had been devoured by the snails.[52] Amalia marginata in captivity has been fed upon the larvae of Euchelia jacobaeae, eating three in two hours.[53]
Limax maximus (Fig. 19) has been seen frequently to make its way into a dairy and feed on raw beef.[54] Individuals kept in confinement are guilty of cannibalism. Mr. W. A. Gain kept three specimens in a box together, and found one of them two-thirds eaten, “the tail left clean cut off, reminding one of that portion of a fish on a fishmonger’s stall.” That starvation did not prompt the crime was proved by the fact that during the preceding night the slug had been supplied with, and had eaten, a considerable quantity of its favourite food. On two other occasions the same observer found one of his slugs deprived of its slime and a portion of its skin, and in a dying condition.[55] An adult L. maximus, kept for thirty-three days in captivity with a young Arion ater, attacked it frequently, denuded it of its slime, and gnawed numerous small pieces of skin off the body and mantle.[56] The present writer has found no better bait for this species on a warm summer night than the bodies of its brethren which were slain on the night preceding; it will also devour dead Helix aspersa. Mr. Gain considers it a very dainty feeder, preferring fungi to all other foods, and apparently doing no harm in the garden.
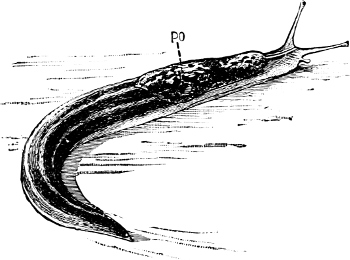
Fig. 19.—Limax maximus L. PO, pulmonary orifice: × ⅔.
[33]
Limax flavus, which is fond of inhabiting the vicinity of cellars, makes its presence most disagreeable by attacking articles of food, and especially by insinuating itself into vessels containing meal and flour.[57] It is particularly partial to cream.
Slugs will sometimes bite their captor’s hands. Mr. Kew relates that a Limax agrestis, on being stopped with the finger, while endeavouring to escape from the attack of a large Arion, attempted to bite fiercely, the rasping action of its radula being plainly felt. According to the same authority, probably all the slugs will rasp the skin of the finger, if it is held out to them, and continue to do so for a considerable time, without however actually drawing blood.[58] While Mr. Gain was handling a large Arion ater, it at once seized one of the folds of skin between the fingers of the hand on which it was placed; after the action of the radula had been allowed to continue for about a minute, the skin was seen to be abraded.[59] Another specimen of Arion ater, carried in the hand for a long time enclosed in a dock leaf, began to rasp the skin. The operation was permitted until it became too painful to bear. Examination with a lens showed the skin almost rasped away, and the place remained tender and sore, like a slight burn, for several days.[60]
Helix pisana, if freshly caught, and placed in a box with other species, will set to work and devour them within twenty-four hours. The present writer has noticed it, in this position, attack and kill large specimens of H. ericetorum, cleaning them completely out, and inserting its elongated body into the top whorls of its unfortunate victims in a most remarkable manner. Amongst a large number of species bred in captivity by Miss F. M. Hele,[61] was Hyalinia Draparnaldi. In the first summer the young offspring were fed on cabbage, coltsfoot, and broadleafed docks. They would not hibernate even in the severest frosts, and, no outdoor food being available, were fed on chopped beef. This, Miss Hele thinks, must have degenerated their appetites, for in the following spring and summer they constantly devoured each other.
Zonites algirus feeds on decayed fruit and vegetables, and on stinking flesh.[62] Achatina panthera has been known to eat[34] meat, other snails (when dead), vegetables, and paper.[63] The common Stenogyra decollata of the South of Europe has a very bad character for flesh-eating habits, when kept in captivity. Mr. Binney[64] kept a number for a long time as scavengers, to clean the shells of other snails. As soon as a living Helix was placed in a box with them, one would attack it, introduce itself into the upper whorls, and completely remove the animal. One day a number of Succinea ovalis were left with them for a short time, and disappeared entirely! The Stenogyra had eaten shell as well as animal. This view of Stenogyra is quite confirmed by Miss Hele, who has bred them in thousands. “I can keep,” she writes,[65] “no small Helix or Bulimus with them, for they at once kill and eat them. They will also eat raw meat.”
Even the common Limnaea stagnalis, which is usually regarded as strictly herbivorous, will sometimes betake itself, apparently by preference, to a diet of flesh. Karl Semper frequently observed the Limnaeae in his aquarium suddenly attack healthy living specimens of the common large water newt (Triton taeniatus), overcome them, and devour them, although there was plenty of their favourite vegetable food growing within easy reach.[66] The same species has also been noticed to devour its own ova, and the larvae of Dytiscus. Limnaea peregra has been detected capturing and partially devouring minnows in an aquarium, when deprived of other food, and Dr. Jeffreys has seen the same species attack its own relatives under similar circumstances, piercing the spire at its thinnest point near to the apex.[67] L. stagnalis, kept in an aquarium, has succeeded in overpowering and partially devouring healthy specimens of the common stickleback.[68]
Powers of Intelligence, Homing, and finding Food.—It is not easy to discover whether land Mollusca possess any faculties which correspond to what we call intelligence, as distinct from their capacities for smell, sight, taste, and hearing. Darwin mentions[69] a remarkable case, communicated to him by Mr. Lonsdale. A couple of Helix pomatia, one of which was sickly,[35] were placed in a small and ill-provided garden. The stronger of the two soon disappeared over the wall into the next garden, which was well furnished with food. It was concluded that the snail had deserted its weakly mate, but after twenty-four hours it returned, and apparently communicated the results of its expedition, for after a short time both started off along the same track, and disappeared over the wall. According to Dr. W. H. Dall,[70] a young girl who possessed a remarkable power over animals succeeded in training a snail (H. albolabris) to come out of its lurking-place at her call. If placed in a room, it would shrink into its shell at the sound of any other voice, but it would always start off in the direction of hers.
Snails and slugs possess to a considerable extent the faculty of ‘homing,’ or returning to the same hiding-place day after day, after their night excursions in search of food. Mr. C. Ashford once marked with a dab of white paint seven Helix aspersa found lurking under a broken flagstone; at 10 P.M. the same evening three had disappeared on the forage; the next morning all were ‘at home.’ The following night at 10 P.M. five were gone out, two being discovered with some difficulty ‘in a small jungle’ six feet away; the next morning six out of the seven were safely beneath the flagstone. According to the same authority, Helix aspersa will find its way across a cinder-path (which it specially detests) to get to its favourite food, and will return by the same way to its old quarters, although it could easily have found new lodgings nearer the food-supply. A snail has been observed to occupy a hole in the brick wall of a kitchen-garden about four feet from the ground. Leaning against the wall, and immediately under the hole, was a piece of wood, the lower end of which rested in a bed of herbs. For months the snail employed this ladder between its food and its home, coming down as soon as it was dark, and retiring to rest during the day.
In greenhouses a slug will forage night after night—as gardeners know to their cost—over the same beat, and will always return to the same hiding-place. Limax flavus has been noticed crawling with great regularity to a sink from a hole near the water-pipe, and keeping to a well-marked circular track. In all probability the scent, either of the desired object of food, or of[36] the creature’s own trail, plays a considerable part in keeping it to the same outward and homeward track, or at least in guiding it back to its hiding-place. Yet even scent is occasionally at fault, for on one occasion a Limax flavus was accustomed to make nightly excursions to some basins of cream, which were kept in a cool cellar. When the basins were removed to a distant shelf, the creature was found the next morning ‘wandering disconsolately’ about in the place where the basins had formerly stood.[71]
A remarkable case of the power of smell, combined with great perseverance on the part of a Helix, is recorded by Furtado.[72] He noticed a Helix aspersa lodged between a column on a verandah and a flower-pot containing a young banana plant, and threw it away into a little court below, and six or seven yards distant. Next morning the snail was in precisely the same place on the flower-pot. Again he threw it away, to the same distance, and determined to notice what happened. Next morning at nine o’clock, the snail was resting on the rail of a staircase leading up to the verandah from the court; in the evening it started again, quickening its space as it advanced, eventually attacking the banana in precisely the same place where it had been gnawed before.
For further instances of the power of smell in snails, see chap. vii.
Slugs have been known to make their way into bee-hives, presumably for the sake of the honey.[73] ‘Sugaring’ the trees at night for moths will often attract a surprising concourse of slugs. Sometimes a particular plant in a greenhouse will become the object of the slugs’ persistent attacks, and they will neglect every other food in order to obtain it. Farfugium grande is one of these favourite foods, “the young leaves and shoots being always eaten in preference to all other plants growing in the houses; where no Farfugiums were kept the slugs nibbled indiscriminately at many kinds.”[74] The flowers of orchidaceous plants exercise a special attraction over slugs, which appear to have some means of discovering when the plants are in bloom. “I have often observed,” says Mr. T. Baines, “that a slug will travel over the surface of a pot in which is growing a Dendrobium[37] nobile, a Cattleya, Vanda, or similar upright plant for a score of times without ever attempting to ascend into the head of the plant unless it is in bloom, in which case they are certain to find their way straight to the flowers; after which they will descend, and return to some favourite hiding-place, often at the opposite end of the house.”[75] Mr. R. Warner has “actually seen many little slugs suspending themselves by slime-threads from the rafters and descending on the spikes of the beautiful Odontoglossum alexandrae; and thus many spikes, thickly wadded round with cotton wool (which the slugs could not travel over), and growing in pots surrounded by water, had been lost.”[76] Perhaps the most singular instance of a liking for a particular food is that related by Mr. E. Step.[77] In a London publishing house, slugs were observed, during a period of nearly twelve months, to have fed almost nightly on the colouring matter in certain bookcovers, and though the trails were often seen over the shelves, and cabbage and lettuce leaves laid down to tempt the creatures, they continued their depredations with impunity for the time above mentioned.
Limnaea peregra has been observed feeding on old fish-heads thrown into a dirty stream, and a large gathering of Limnaea stagnalis has been noticed feeding upon an old newspaper in a pond on Chislehurst Common, ‘so that for the space of about a square foot nothing else could be seen.’[78]
Tenacity of Life.—Land Mollusca have been known to exhibit, under unusual conditions, remarkable tenacity of life. Some of the most noteworthy and best authenticated instances of this faculty may be here mentioned.
The well-known story of the British Museum snail is thus related by Mr. Baird.[79] On the 25th March 1846 two specimens of Helix desertorum, collected by Charles Lamb, Esq., in Egypt some time previously, were fixed upon tablets and placed in the collection among the other Mollusca of the Museum. There they remained fast gummed to the tablet. About the 15th March 1850, having occasion to examine some shells in the same case, Mr. Baird noticed a recently formed epiphragm over the mouth of one of these snails. On removing the snails from the tablet[38] and placing them in tepid water, one of them came out of its shell, and the next day ate some cabbage leaf. A month or two afterwards it began repairing the lip of its shell, which was broken when it was first affixed to the tablet.
While resident in Porto Santo, from 27th April to 4th May 1848, Mr. S. P. Woodward[80] collected a number of Helices and sorted them out into separate pill-boxes. On returning home, these boxes were placed in empty drawers in an insect cabinet, and on 19th October 1850, nearly two and a half years afterwards, many of them were found to be still alive. A whole bagful of H. turricula, collected on the Ilheo de Cima on 24th April 1849, were all alive at the above-mentioned date.
In September 1858 Mr. Bryce Wright sent[81] to the British Museum two specimens of H. desertorum which had been dormant for four years. They were originally collected in Egypt by a Mr. Vernèdi, who, in May 1854, while stopping at one of the stations in the desert, found a heap of thorn-bushes lying in a corner of the building, rather thickly studded with the snails. He picked off fifteen or twenty specimens, which he carried home and locked up in a drawer, where they remained undisturbed until he gave two to Mr. Wright in September 1858.
In June 1855 Dr. Woodward placed specimens of H. candidissima and H. aperta in a glass box, to test their tenacity of life; he writes of their being still alive in April 1859.
Mr. R. E. C. Stearns records[82] a case of Buliminus pallidior and H. Veatchii from Cerros I. living without food from 1859 to March 1865.
H. Aucapitaine mentions[83] a case of H. lactea found in calcinated ground in a part of the Sahara heated to 122° F., where no rain was said to have fallen for five years. The specimen revived after being enclosed in a bottle for three and a half years.
In August 1863, Mr. W. J. Sterland[84] put specimens of H. nemoralis in a box and afterwards placed the box in his cabinet; in November 1866 one specimen was discovered to be alive.
Gaskoin relates[85] a case in which specimens of H. lactea were purchased from a dealer in whose drawer they had been for two[39] years. This dealer had them from a merchant at Mogador, who had kept them for more than that time under similar conditions. One of these shells on being immersed in water revived, and in April 1849 was placed quite alone under a bell jar with earth and food. In the end of the following October about thirty young H. lactea were found crawling on the glass.
Mr. R. D. Darbishire bought[86] some H. aperta in the market at Nice on 18th February 1885. Two specimens of these, placed with wool in a paper box, were alive in December 1888. This is a very remarkable case, H. aperta not being, like H. desertorum, H. lactea, H. Veatchii and Bul. pallidior, a desert snail, and therefore not accustomed to fasting at all.
Age of Snails.—It would appear, from the existing evidence, which is not too plentiful, that five years is about the average age of the common garden snail. Mr. Gain has published[87] some interesting observations on the life of a specimen from the cradle to the grave, which may be exhibited in a tabular form.
| Aug. 1882. | Eggs hatched; one attained diameter of ⅜ in. before winter; fed on coltsfoot and cabbage. |
| 5th Oct. 1883. | Shell 1 in. in diameter, no lip formed. |
| July 1884. | Shell finished; diameter 1⅛ in., including perfect lip. |
| 3rd May 1885. | Left winter quarters; companion introduced, with which it was seen in company on 5th August. |
| 9th Aug. „ | Laid eggs in soil, which were hatched on 10th September, and feeding on 17th September; in May 1886 the largest of these was 11/16 in. diameter. |
| 13th Oct. 1887. | Old snail died, aged 5 years 2 months. |
According to Clessin, the duration of life in Vitrina is one year, Cyclas 2 years; Hyalinia, Succinea, Limnaea, Planorbis, and Ancylus are full grown in 2 to 3 years, Helix and Paludina in 2 to 4, and Anodonta in 12 to 14. Hazay finds[88] that the duration of life in Hyalinia is 2 years, in Helix pomatia 6 to 8, in Helix candicans 2 to 3, in Paludina 8 to 10, in Limnaea and Planorbis 3 to 4.
[40]
Growth of the Shell.—Mr. E. J. Lowe, many years ago, conducted[89] some interesting experiments on the growth of snails. The facts arrived at were—
(1) The shells of Helicidae increase but little for a considerable period, never arriving at maturity before the animal has once become dormant.
(2) Shells do not grow whilst the animal itself remains dormant.
(3) The growth of shells is very rapid when it does take place.
(4) Most species bury themselves in the ground to increase the dimensions of their shells.
Six recently hatched H. pomatia were placed in a box and regularly fed on lettuce and cabbage leaves from August until December, when they buried themselves in the soil for winter; at this period they had gradually increased in dimensions to the size of H. hispida. On the 1st April following, the box was placed in the garden, and on the 3rd the Helices reappeared on the surface, being no larger in size than they were in December. Although regularly fed up to 20th June, they were not perceptibly larger, but on that day five of them disappeared, having buried themselves, with the mouth of the shell downwards, in the soil. After ten days they reappeared, having in that short time grown so rapidly as to be equal in size to H. pisana. On the 15th July they again buried themselves, and reappeared on 1st August, having again increased in size. For three months from this date they did not become perceptibly larger; on 2nd November food was withheld for the winter and they became dormant.
A similar experiment, with similar results, was carried on with a number of H. aspersa, hatched on 20th June. During the summer they grew but little, buried themselves on 10th October with the head upwards, and rose to the surface again on 5th April, not having grown during the winter. In May they buried themselves with the head downwards, and appeared again in a week double the size; this went on at about fortnightly intervals until 18th July, when they were almost fully grown.
Helix nemoralis, H. virgata, H. caperata, and H. hispida bury[41] themselves to grow; H. rotundata burrows into decayed wood; Hyalinia radiatula appears to remain on decaying blades of grass; Pupa umbilicata, Clausilia rugosa, and Buliminus obscurus bury their heads only.
The observations of Mr. W. E. Collinge[90] do not at all agree with those of Mr. Lowe, with regard to the mode in which land Mollusca enlarge their shells. He bred and reared most of the commoner forms of Helix and also Clausilia rugosa, but never saw them bury any part of their shell when enlarging it. While admitting that they may increase their shells when in holes or burrows of earthworms, he thinks that the process of burying would seriously interfere with the action of the mantle during deposition, and in many cases damage the membranaceous film before the calcareous portion was deposited. Mr. Collinge has found the following species under the surface in winter: Arion ater (3–4 in.), Agriolimax agrestis, (6–8 in.), Hyalinia cellaria and H. alliaria (6–8 in.), Hyalinia glabra (5 in.), Helix aspersa (5–6 in.), H. rufescens (4–6 in.), H. rotundata (4–5 in.), H. hispida (7 in.), Buliminus obscurus (4–6 in.), B. montanus[91] (24 in.), and the following in summer, Hyalinia cellaria and alliaria (6–8 in.), Helix rotundata (4–5 in.), Balea perversa (6–8 in.), Cyclostoma elegans (3–4 in.). The same author has found the following species of fresh-water Mollusca living in hard dry mud: Sphaerium corneum (3–14 in.), S. rivicola (5–6 in.), S. lacustre (10–14 in.), all the British species of Pisidium (4–12 in.), Limnaea truncatula (18 in., a single specimen). All our species of Unio, Anodonta, Bithynia, and Paludina bury themselves habitually in fine or thick wet mud, to a depth of from 4 to 14 inches.
This burying propensity on the part of Mollusca has been known to play its part in detecting fraud. When my friend Mr. E. L. Layard was administering justice in Ceylon, a native landowner on a small scale complained to him of the conduct of his neighbour, who had, during his absence from home, diverted a small watercourse, which ran between their holdings, in such a way as to filch a certain portion of the land. The offender had filled up and obliterated the ancient course of the stream, and protested that it had never run but in its present bed.[42] Mr. Layard promptly had a trench sunk across what was said to be the old course, and the discovery of numerous living Ampullaria, buried in the mud, confirmed the story of one of the litigants and confounded the other.[92]
Depositing and Hatching of Eggs: Self-fertilisation.—There appears to be no doubt that Helices, when once impregnated, can lay successive batches of eggs, and possibly can continue laying for several years, without a further act of union. A specimen of Helix aspersa was noticed in company with another on 5th August; on 9th August it laid eggs in the soil, and early in the following summer it laid a second batch of eggs, although its companion had been removed directly after its first introduction. An Arion received from a distance laid 30 eggs on 5th September, and 70 more on the 23rd of the same month, although quite isolated during the whole time.[93] By far the most remarkable case of the kind is related by Gaskoin.[94] A specimen of Helix lactea was kept in a drawer for about two years, and then in another drawer for about two years more. It was then taken out, and placed in water, when it revived, and was placed alone under a bell jar with earth and food. Six months after, about 30 young H. lactea were found crawling on the glass, the act of oviposition not having been observed.
The observations of Mr. F. W. Wotton,[95] with regard to the fertilisation and egg-laying of Arion ater, are of extreme interest and value. A pair of this species, kept in captivity, united on 10th September 1889, the act lasting about 25 minutes. From that date until the eggs were laid, the animals looked sickly, dull of colour, with a somewhat dry skin. Eggs were deposited in batches, one, which we will call A, beginning three days before B. On 10th October A laid 80 eggs; on the 16th, 110; on the 25th, 77; on 8th November, 82; and on 17th November, 47; making a total of 396. Specimen B, which began on 13th October, three days after A, made up for the delay by laying 246 eggs in 40 hours; on 26th October it laid 9, on 10th November, 121; and on 30th November, 101; a total of[43] 477. These eggs weighed 624 to the ounce, and, in excluding the batch of 246, B parted with ⅜ of its own weight in 40 hours, while the whole number laid were rather over ¾ of its own weight!
While depositing the eggs, the slug remained throughout in the same position on the surface of the ground, with the head drawn up underneath the mantle, which was lifted just above the reproductive orifice. When taken into the hand, it went on laying eggs without interruption or agitation of any kind. After it had finished laying it ate half a raw potato and then took a bath, remaining submerged for more than an hour. Bathing is a favourite pastime at all periods. Specimens, says Mr. Wotton, have survived a compulsory bath, with total submersion, of nearly three days’ duration.
Mr. Wotton’s account of the hatching of the eggs is equally interesting. It is noticeable that the eggs of one batch do not hatch by any means simultaneously; several days frequently intervene. The average period is about 60 days, a damp and warm situation bringing out the young in 40 days, while cold and dryness extended the time to 74 days, extremes of any kind proving fatal. Of the batch of eggs laid by B on 30th November, the first 2 were hatched on the following 16th January, and 2 more on the 17th; others, from 10 to 20, followed suit on the succeeding 5 days, until 82 in all were hatched, the remaining 19 being unproductive.[96]
By placing the egg on a looking-glass the act of exclusion can be perfectly observed. For several days the inmate can be seen in motion, until at last a small crack appears in the surface of the shell: this gradually enlarges, until the baby slug is able to crawl out, although it not unfrequently backs into the shell again, as if unwilling to risk itself in the world. When it once begins to crawl freely, it buries itself in the ground for 4 or 5 days without food, after which time it emerges, nearly double its original size. At exclusion, the average length is 9 mm., increasing to 56 mm. after the end of 5 months. Full growth is attained about the middle of the second year, and nearly all die at the end of this year or the beginning of the next. Death from exhaustion frequently occurs after parturition. Death[44] from suffocation is sometimes the result of the formation of small blisters on the margin of the respiratory aperture. The attacks of an internal parasite cause death in a singular way. The upper tentacles swell at the base in such a way as to prevent their extrusion; digestive troubles follow, with rigidity and loss of moisture, and death ensues in 2 or 3 days.
Mr. Wotton isolated newly-hatched specimens, with the view of experimenting on their power of self-fertilisation, if the opportunity of fertilising and being fertilised by others was denied them. One of these, after remaining in absolute solitude for 10½ months, began to lay, scantily at first (11th January, 2; 25th January, 2; 11th February, 2), but more abundantly afterwards (3rd April, 60; 15th and 16th, 70; 29th, 53, etc.), the eggs being hatched out in 42–48 days. The precautions taken seem to have been absolutely satisfactory, and the fact of the power of self-fertilisation appears established as far as Arion ater is concerned.
Braun took young individuals of Limnaea auricularia on the day they were hatched out, and placed them singly in separate vessels with differing amounts of water. This was on 15th June 1887. In August 1888 specimen A had only produced a little spawn, out of which three young were hatched; specimen B had produced four pieces of spawn of different sizes, all of which were hatched; specimen C, which happened to be living with three Planorbis, produced five pieces of spawn distinctly Limnaeidan, but nothing is recorded of their hatching. Self-impregnation, therefore, with a fruitful result, appears established for this species of Limnaea.[97]
Reproduction of Lost Parts.—When deprived of their tentacles, eyes, or portions of the foot, Mollusca do not seem to suffer severely, and generally reproduce the lost parts in a short time. If, however, one of the ganglia is injured, they perish. Certain of the Mollusca possess the curious property of being able to amputate certain parts at will. When Prophysaon, a species of Californian slug, is annoyed by being handled, an indented line appears at a point about two-thirds of the length from the head, the line deepens, and eventually the tail is shaken completely off. Sometimes the Prophysaon only threatens this spontaneous dismemberment; this line appears (always exactly[45] in the same place), but it thinks better of it, and the indentation proceeds no further.[98] According to Gundlach,[99] Helix imperator and H. crenilabris, two large species from Cuba, possess the same property, which is said to be also characteristic of the sub-genus Stenopus (W. Indies). Amongst marine species, Harpa ventricosa and Solen siliqua have been observed to act in a similar way, Harpa apparently cutting off the end of the foot by pressure of the shell. Karl Semper, in commenting on the same property in species of Helicarion from the Philippines (which whisk their tail up and down with almost convulsive rapidity, until it drops off), considers[100] it greatly to the advantage of the mollusc, since any predacious bird which attempted to seize it, but only secured a fragment of tail, would probably be discouraged from a second attack, especially as the Helicarion would meanwhile have had time to conceal itself among the foliage.
Strength and Muscular Force.—The muscular strength of snails is surprisingly great. Sandford relates[101] an experiment on a Helix aspersa, weighing ¼ oz. He found it could drag vertically a weight of 2¼ oz., or nine times its own weight. Another snail, weighing ⅓ oz., was able to drag in a horizontal direction along a smooth table twelve reels of cotton, a pair of scissors, a screwdriver, a key, and a knife, weighing in all no less than 17 oz., or more than fifty times its own weight. This latter experiment was much the same as asking a man of 12 stone to pull a load of over 3¾ tons.
If a snail be placed on a piece of glass and made to crawl, it will be seen that a series of waves appear to pursue one another along the under surface of the foot, travelling from back to front in the direction in which the animal is moving. Simroth has shown that the sole of the foot is covered with a dense network of muscular fibres, those which run longitudinally being chiefly instrumental in producing the undulatory motion. By means of these muscles the sole is first elongated in front, and then shortened behind to an equal extent. Thus a snail slides, not on the ground, but on its own mucus, which it deposits mechanically, and which serves the purpose of lubricating the[46] ground on which it travels. It has been calculated that an averaged sized snail of moderate pace progresses at the rate of about a mile in 16 days 14 hours.[102]
Sudden Appearance of Mollusca.—It is very remarkable to notice how suddenly Pulmonata seem to appear in certain districts where they have not been noticed before. This sudden appearance is more common in the case of fresh-water than of land Mollusca, and there can be little doubt that, wherever a new pond happens to be formed, unless there is something in its situation or nature which is absolutely hostile to molluscan life, Mollusca are certain to be found in it sooner or later. “Some 23 years ago,” writes Mr. W. Nelson,[103] “I was in the habit of collecting shells in a small pond near to the Black Hills, Leeds. At that time the only molluscan forms found there were a dwarf form of Sphaerium lacustre, Pisidium pusillum, Planorbis nautileus, and Limnaea peregra. About 10 years ago I resumed my visits to the locality, and found, in addition to the species already enumerated, Planorbis corneus. These were the only species found there until this spring [1883], when, during one of my frequent visits, I was surprised to find Physa fontinalis and Planorbis vortex were added to the growing list of species. Later on Pl. carinatus, Limnaea stagnalis, and Ancylus lacustris turned up; and during June, Pl. contortus was found in this small but prolific pond.” Limnaea glutinosa is prominent for these remarkable appearances and disappearances. In 1822 this species suddenly appeared in some small gravel pits at Bottisham, Cambs., in such numbers that they might have been scooped out by handfuls. After that year they did not appear numerous, and after three or four seasons they gradually disappeared.[104] Physa (Aplecta) hypnorum is noted in a similar way. In February 1852, for instance, after a wet month, the water stood in small puddles about 3 feet by 2 in a particular part of Bottisham Park which was sometimes a little swampy, though usually quite dry. One of these puddles was found to contain immense numbers of the Aplecta, which up to that time had not been noted as occurring in Cambridgeshire at all.[105] In a few days the species entirely disappeared and was never again noticed in the locality.[106]
[47]
Writing to the Zoological Society of London from New Caledonia, Mr. E. L. Layard remarks:[107] “The West Indian species Stenogyra octona has suddenly turned up here in thousands; how introduced, none can tell. They are on a coffee estate at Kanala on the east coast. I have made inquiries, and cannot find that the planter ever had seed coffee from the West Indies. All he planted came from Bombay, and it would be interesting to find out whether the species has appeared there also.”
Sometimes a very small event is sufficient to disturb the natural equilibrium of a locality, and to become the cause either of the introduction or of the destruction of a species. In 1883 a colony of Helix sericea occupied a portion of a hedge bottom twenty yards long near Newark. It scarcely occurred outside this limit, but within it was very plentiful, living in company with H. nemoralis, H. hortensis, H. hispida, H. rotundata, Hyalinia cellaria and Hy. nitidula, and Cochlicopa lubrica. In 1888 the hedge was well trimmed, but the bottom was not touched, and the next year a long and careful search was required to find even six specimens of the sericea.[108]
Showers of Shells.—Helix virgata, H. caperata, and Cochlicella acuta sometimes occur on downs near our sea-coasts in such extraordinary profusion, that their sudden appearance out of their hiding-places at the roots of the herbage after a shower of rain has led to the belief, amongst credulous people, that they have actually descended with the rain. There seems, however, no reason to doubt that Mollusca may be caught up by whirlwinds into the air and subsequently deposited at some considerable distance from their original habitat, in the same way as frogs and fishes. A very recent instance of such a phenomenon occurred[109] at Paderborn, in Westphalia, where, on 9th August 1892, a yellowish cloud suddenly attracted attention from its colour and the rapidity of its motion. In a few moments it burst, with thunder and a torrential rain, and immediately afterwards the pavements were found to be covered with numbers of Anodonta anatina, all of which had the shell broken by the violence of the fall. It was clearly established that the shells[48] could not have been washed into the streets from any adjacent river or pond, and their true origin was probably indicated when it was found that the funnel-shaped cloud which burst over the town had passed across the one piece of water near Paderborn, which was known to contain the Anodonta in abundance.
Cases of Singular Habitat.—Mollusca sometimes accustom themselves to living in very strange localities, besides the extremes of heat and cold mentioned above (pp. 23–24). In the year 1852, when some large waterpipes in the City Road, near St. Luke’s Hospital, were being taken up for repairs, they were found to be inhabited in considerable numbers by Neritina fluviatilis and a species of Limnaea.[110] Dreissensia polymorpha has been found in a similar situation in Oxford Street, and also in Hamburg, and has even been known to block the pipes and cisterns of private houses. In an engine cistern at Burnley, 60 feet above the canal from which the water was pumped into the cistern, were found the following species: Sphaerium corneum, S. lacustre; Valvata piscinalis, Bithynia tentaculata; Limnaea peregra, very like Succinea in form and texture; Planorbis albus, P. corneus, P. nitidus, P. glaber, and thousands of P. dilatatus, much larger than the forms in the canal below, a fact probably due to the equable temperature of the water in the cistern all the year round.[111] In certain parts of southern Algeria the fresh-water genera Melania and Melanopsis inhabit abundantly waters so surcharged with salt that the marine Cardium edule has actually become extinct from excess of brine. The common Mytilus edulis is sometimes found within the branchial chamber and attached to the abdomen of crabs (Carcinus maenas), which are obliged to carry about a burden of which they are powerless to rid themselves (see p. 78). A variety of the common Limnaea peregra lives in the hot water of some of the geysers of Iceland, and has accordingly been named geisericola.
Underground Snails.—Not only do many of the land Mollusca aestivate, or hibernate, as the case may be, beneath the surface of the soil, but a certain number of species live permanently underground, like the mole, and scarcely ever appear in the light of day. Our own little Caecilianella acicula lives[49] habitually from 1 to 3 feet below ground, appearing to prefer the vicinity of graveyards. Testacella, the carnivorous slug, scarcely ever appears on the surface during the day, except when driven by excessive rain, and even then it lurks awhile under some protecting cover of leafage. There is a curious little Helix (tristis Pfr.), peculiar to Corsica, which is of distinctly subterranean habits. It lives in drifted sand above high-water mark, always at the roots of Genista Saltzmanni, at a depth which varies with the temperature and dryness of the air. In hot and very dry weather it buries itself nearly 2 feet below the surface, only coming up during rain, and burying itself again immediately the rain is over. Like a Solen, it often has a hole above its burrow, by which it communicates with the air above, so as to avoid being stifled in the sand. The animal, in spite of its dry habitat, is singularly soft and succulent, and exudes a very glutinous mucus. It probably descends in its burrow until it arrives at the humid stratum, the persistence of which is due to the capillarity of the sand.[112] I am assured by Mr. E. L. Layard that precisely similar underground habits are characteristic of Coeliaxis Layardi, which lives exclusively in sand at the roots of scrub and coarse grass at East London.
Rock-boring Snails.—Cases have sometimes been recorded, from which it would appear that certain species of snails possess the power of excavating holes in rocks to serve as hiding-places. At Les Bois des Roches, ten miles from Boulogne, occur a number of solid calcareous rocks scattered about in the wood. The sides of the rocks which face N.E. and E. are covered with multitudes of funnel-shaped holes, 1½ inch in diameter at the opening and contracting suddenly within to ½ inch. Sometimes the holes are 6 inches deep, and terminate, after considerable windings, in a cup-shaped cavity. Helix hortensis inhabits these holes, and has been observed to excavate them at the rate of ½ inch each hibernation, choosing always the side of the rock which is sheltered from the prevailing rains. It does not form an epiphragm, but protrudes part of its body against the rock. That the snails secrete an acid which acts as a solvent seems probable from the fact that red litmus paper, on being applied to the place where the foot has been, becomes stained with violet.[113][50] Helix aspersa is said to excavate holes 10 to 12 cm. deep at Constantine,[114] and H. Mazzullii is recorded as perforating limestone at Palermo.[115]
Snails as Barometers.—An American writer of more than thirty years ago[116] gave his experience of Helices as weather-prophets. According to him, H. alternata is never seen abroad except shortly before rain; it then climbs on the bark of trees, and stations itself on leaves. Helix clausa, H. ligera, H. pennsylvanica, and H. elevata climb trees two days before rain, if it is to be abundant and continuous. Succinea does the same, and its body is yellow before rain and bluish after it. Several of the Helices assume a sombre colour after rain, when their bodies are exceedingly humid; after the humidity has passed off they resume a clearer and lighter tint.
Production of Musical and other Sounds.—Certain molluscs are said to be capable of producing musical sounds. Sir J. E. Tennent describes his visit to a brackish-water lake at Batticaloa, in Ceylon, where the fishermen give the name of the ‘crying shell’ to the animal supposed to produce the sounds. “The sounds,” he says,[117] “came up from the water like the gentle thrills of a musical chord, or the faint vibrations of a wineglass when its rim is rubbed by a moistened finger. It was not one sustained note, but a multitude of tiny sounds, each clear and distinct in itself; the sweetest treble mingling with the lowest bass. On applying the ear to the woodwork of the boat, the vibration was greatly increased in volume. The sounds varied considerably at different points as we moved across the lake, and occasionally we rowed out of hearing of them altogether.” According to the fishermen, the shells were Pyrazus palustris and Littorina laevis. It appears uncertain whether the sounds are really due to Mollusca. Fishermen in other parts of India assert that the sounds are made by fish, and, like those in Ceylon, produce the fish which they say ‘sings.’ The same, or a similar sound, has also been noticed to issue from the water in certain parts of Chili, and on the northern shores of the Gulf of[51] Mexico. Dendronotus arborescens, when confined in a glass jar of sea water, has been noticed[118] to emit a sound like the clink of a steel wire. According to Lieut.-Col. Portlock,[119] F.R.S., Helix aperta, a very common species in South Europe, has the property of emitting sounds when irritated. When at Corfu, he noticed that if the animal is irritated by a touch with a piece of straw or other light material, it emits a noise, as if grumbling at being disturbed. He kept a specimen in his house for a considerable time, which would make this noise whenever it was touched.
The Rev. H. G. Barnacle describes the musical properties of Achatinella in the following terms:[120] “When up the mountains of Oahu I heard the grandest but wildest music, as from hundreds of Aeolian harps, wafted to me on the breezes, and my companion (a native) told me it came from, as he called them, the singing shells. It was sublime. I could not believe it, but a tree close at hand proved it. On it were many of the Achatinella, the animals drawing after them their shells, which grated against the wood and so caused a sound; the multitude of sounds produced the fanciful music. On this one tree I took 70 shells of all varieties.”
Habits of the Agnatha.—Not much is known of the habits and mode of life of the Agnatha, or carnivorous Land Mollusca. In this country we have only two, or at most three, of this group, belonging to the genus Testacella, and, in all probability, not indigenous to our shores. There seems little doubt, when all the circumstances of their discovery are taken into account, that both Testacella haliotidea and T. Maugei have been imported, perhaps from Spain or Portugal in the first instance, along with roots imbedded in foreign earth, for their earliest appearances can almost invariably be traced back to the neighbourhood of large nursery grounds, or else to gardens supplied directly from such establishments.
The underground life of Testacella makes observation of its habits difficult. It is believed to feed exclusively on earthworms, which it pursues in their burrows. Continued wet weather drives it to the surface, for though loving damp soil it[52] is decidedly averse to too much moisture, and under such circumstances it has even been noticed[121] in considerable numbers crawling over a low wall. In the spring and autumn months, according to Lacaze-Duthiers,[122] it comes to the surface at night, hiding itself under stones and débris during the day. Earthworms are, at these periods, nearer the surface, and the Testacella has been seen creeping down into their burrows. The author has taken T. Maugei abundantly under clumps of the common white pink in very wet weather, lying in a sort of open nest in the moist earth. On the other hand, when the earth is baked dry by continued drought, they either bury themselves deeper, sometimes at a depth of 3 feet, in the ground, or else become encysted in a capsule of hardened mucus to prevent evaporation from the skin. When first taken from the earth and placed in a box, the Testacella invariably resents its capture by spitting up the contents of its stomach in the shape of long fragments of half-digested worms.
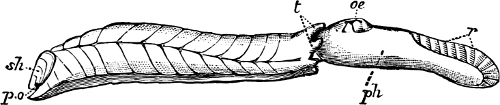
Fig. 20.—Testacella haliotidea Drap., protruding its pharynx (ph) and radula (r); oe, oesophagus; p.o, pulmonary orifice; sh, shell; t, tentacles (after Lacaze-Duthiers).
It appears not to bite the worm up before swallowing it, but contrives, in the most remarkable manner, to take down whole worms apparently much too large for its stomach. Mr. Butterell relates[123] that, after teasing a specimen of T. Maugei, and making it emit a quantity of frothy mucus from the respiratory aperture, he procured a worm of about three inches long, and rubbed the worm gently across the head of the Testacella. The tongue was rapidly extended, and the victim seized. The odontophore was then withdrawn, carrying with it the struggling worm, which made every effort to escape, but in vain; in about five minutes all had disappeared except the head, which was rejected. This protrusion of the tongue (radula) and indeed of the whole[53] pharynx, is a very remarkable feature in the habits of the animal. It appears, as it were, to harpoon its prey by a rapid thrust, and when the victim is once pierced by a few of the powerful sickle-shaped teeth (compare chap. viii.) it is slowly but surely drawn into the oesophagus (Fig. 20).
Most gardeners are entirely ignorant of the character of Testacella, and confuse it, if they happen to notice it at all, with the common enemies of their tender nurslings. Cases have been known, however, when an intelligent gardener has kept specimens on purpose to kill worms in ferneries or conservatories. In some districts these slugs are very numerous; Lacaze-Duthiers once dug 182 specimens from a good well-manured piece of ground whose surface measured only ten square yards.
Towards the end of September or beginning of October the period of hibernation begins. I infer this from the behaviour of specimens kept in captivity, which, for about a fortnight before this time, gorged themselves inordinately on as many worms as I chose to put into their box, and then suddenly refused food, buried themselves deeply in the earth, and appeared no more during the winter. The eggs are apparently much less numerous than is the case with Limax or Helix, and very large, measuring about ⅙ inch in diameter. They are enveloped in a remarkably tough and elastic membrane, and, if dropped upon any hard surface, rebound several inches, just like an india-rubber ball.
The animal creeps rather rapidly, and has the power of elongating its body to a remarkable extent. When placed on the surface of the ground, in the full light of day, it soon betrays uneasiness, and endeavours to creep into concealment. Its method of burying itself is very interesting to watch. It first elongates its neck and inserts its head into the soil; gradually the body begins to follow, while the tail tilts upwards into the air. No surface motion of the skin, no writhing or wriggling motion of any kind occurs; the creature simply works its way down in a stealthy and mysterious way, until at last it is lost to view.
The great Glandina, which attain their maximum development in Mexico and the southern United States, are a very noticeable family in this group. According to Mr. Binney,[124] Glandina truncata Gmel., one of the commonest species of the genus, is somewhat aquatic in its habits. It is found in the sea[54] islands of Georgia and around the keys and everglades of Florida, where it attains a maximum length of 4 inches, while in less humid situations it scarcely measures more than 1 inch. It occurs most abundantly in the centre of clumps and tussocks of coarse grass in marshes close to the sea-coast. By the action of the sharp, sickle-shaped teeth of its radula the soft parts of its prey (which consists chiefly of living Helices) are rapidly rasped away; sometimes they are swallowed whole. It has been known to attack Limax when confined in the same box, rasping off large pieces of the integument. In one case an individual was noticed to devour one of its own species, thrusting its long neck into the interior of the shell, and removing all the viscera.

Fig. 21.—Glandina sowerbyana Pfr. (Strebel).
The Glandinae of southern Europe, although scarcely rivalling those of Central America in size or beauty, possess similar carnivorous propensities. Glandina Poireti has been observed,[125] on Veglia Island, attacking a living Cyclostoma elegans. By its powerful teeth it filed through two or three whorls of the shell of its victim, and then proceeded to devour it, exactly in the same manner as a Natica or Buccinum perforates the shell of a Tellina or Mactra in order to get at its contents.
Few observations appear to have been made on the habits or food of Streptaxis, Rhytida, Ennea, Daudebardia, Paryphanta, and other carnivorous Mollusca. A specimen of Ennea sulcata, enclosed in the same box as a Madagascar Helix (sepulchralis Fér.) many times its own size, completely emptied the shell of its inhabitant.[126] Mr. E. L. Layard informs me that certain Cape Rhytida, e.g. R. capsula Bens., R. dumeticola Bens., and R. vernicosa Kr., eat Cyclostoma affine, Helix capensis, H. cotyledonis, etc. To Mr. Layard I am also indebted for the—perhaps apocryphal—tradition that the best time to capture the great[55] Aerope caffra Fér. in numbers was after an engagement between the Kaffirs and Zulus, when they might be observed streaming from all points of the compass towards the field of slaughter. The Cuban Oleacina are known to secrete a very bitter fluid which they emit; this perhaps produces a poisoning or benumbing effect upon their victims when seized. They devour operculates, e.g. Helicina regina and sagraiana.[127]
[56]
The juicy flesh and defenceless condition of many of the Mollusca make them the favourite food and often the easy prey of a host of enemies besides man. Gulls are especially partial to bivalves, and may be noticed, in our large sandy bays at the recess of the tide, busily devouring Tellina, Mactra, Mya, Syndosmya, and Solen. On the Irish coast near Drogheda a herring gull has been observed[128] to take a large mussel, fly up with it in the air over some shingly ground and let it fall. On alighting and finding that the shell was unbroken it again took it up and repeated the process a number of times, flying higher and higher with it until the shell was broken. Hooded crows, after many unavailing attempts to break open mussels with their beak, have been seen to behave in a similar way.[129] Crows, vultures, and aquatic birds carry thousands of mussels, etc., up to the top of the mountains above Cape Town, where their empty shells lie in enormous heaps about the cliffs.[130]
The common limpet is the favourite food of the oyster-catcher, whose strong bill, with its flattened end, is admirably calculated to dislodge the limpet from its seat on the rock. When the limpet is young, the bird swallows shell and all, and it has been calculated that a single flock of oyster-catchers, frequenting a small Scotch loch, must consume hundreds of[57] thousands of limpets in the course of a single year. Rats are exceedingly fond of limpets, whose shells are frequently found in heaps at the mouth of rat holes, especially where a cliff shelves gradually towards a rocky shore. A rat jerks the limpet off with a sudden movement of his powerful jaw, and, judging from the size of the empty shells about the holes, has no difficulty in dislodging the largest specimens. ‘I once landed,’ relates a shepherd to Mr. W. Anderson Smith,[131] ‘on the I. of Dunstaffnage to cut grass, and it was so full of rats that I was afraid to go on; and the grass was so full of limpets that I could scarcely use the scythe, and had to keep sharpening it all the time.’ Sometimes, however, the limpet gets the better both of bird and beast. The same writer mentions the case of a rat being caught by the lip by a limpet shell, which it was trying to dislodge. A workman once observed[132] a bird on Plymouth breakwater fluttering in rather an extraordinary manner, and, on going to the spot, found that a ring dotterel had somehow got its toe under a limpet, which, in closing instantly to the rock, held it fast. Similar cases of the capture of ducks by powerful bivalves are not uncommon, and it is said that on some parts of the American coasts, where clams abound, it is impossible to keep ducks at all,[133] for they are sure to be caught by the molluscs and drowned by the rising tide.
The Weekly Bulletin of San Francisco, 17th May 1893, contains an account of the trapping of a coyote, or prairie wolf, at Punta Banda, San Diego Co., by a Haliotis Cracherodii. The coyote had evidently been hunting for a fish breakfast, and finding the Haliotis partially clinging to the rock, had inserted his muzzle underneath to detach it, when the Haliotis instantly closed down upon him and kept him fast prisoner.
Rats devour the ponderous Uniones of North America. When Unio moves, the foot projects half an inch or more beyond the valves. If, when in this condition, the valves are tightly pinched, the foot is caught, and if the pinching is continued the animal becomes paralysed and unable to make use of the adductor muscles, and consequently flies open even if the pressure is relaxed. The musk-rat (Fiber zibethicus) seizes the Unio in his jaws, and by the time he reaches his hole, the Unio[58] is ready to gape.[134] Rats also eat Vivipara, and even Limnaea, in every part of the world.
Every kind of slug and snail is eaten greedily by blackbirds, thrushes, chaffinches, and in fact by many species of birds. A thrush will very often have a special sacrificial stone, on which he dashes the shells of Helix aspersa and nemoralis, holding them by the lip with his beak, until the upper whorls are broken; heaps of empty shells will be found lying about the place of slaughter. The bearded Titmouse (Parus biarmicus) consumes quantities of Succinea putris and small Pupa, which are swallowed whole and become triturated in the bird’s stomach by the aid of numerous angular fragments of quartz.[135]
Frogs and toads are very partial to land Mollusca. A garden attached to the Laboratory of Agricultural Chemistry at Rouen had been abandoned for three years to weeds and slugs. The director introduced 100 toads and 90 frogs, and in less than a month all the slugs were destroyed, and all kinds of vegetables and flowers, whose cultivation had until then been impossible, were enabled to flourish.[136]
Certain Coleoptera are known to prey upon Helices and other land Mollusca. Récluz noticed, near Agde, a beetle (Staphylinus olens) attack Helix ericetorum when crawling among herbage, sticking its sharp mandibles into its head. Every time the snail retreated into its shell the beetle waited patiently for its reappearance, until at last the snail succumbed to the repeated assaults. M. Lucas noticed, at Oran, the larva of a Drilus attacking a Cyclostoma. The Drilus stood sentinel at the mouth of a shell, which was closed by the operculum, until the animal began to issue forth. The Drilus then with its mandibles cut the muscle which attaches the operculum to the foot, disabling it sufficiently to prevent its being securely closed, upon which it entered and took possession of the body of its defenceless host, completing its metamorphosis inside the shell, after a period of six weeks.[137] The female glow-worm (Lampyris noctiluca) attacks and kills Helix nemoralis.
Among the Clavicornia, some species of Silpha carry on a determined warfare against small Helices. They seize the shell[59] in their mandibles, and then, throwing their head backwards, break the shell by striking it against their prothorax.
The common water beetle, Dytiscus marginalis, from its strength and savage disposition, is a dangerous enemy to fresh-water Mollusca. One Dytiscus, kept in an aquarium, has been noticed to kill and devour seven Limnaea stagnalis in the course of one afternoon. The beetles also eat L. peregra, but apparently prefer stagnalis, for when equal quantities of both species were placed within their reach, they fixed on the latter species first.[138]
In East Africa a species of Ichneumon (Herpestes fasciatus) devours snails, lifting them up in its forepaws and dashing them down upon some hard substance.[139] In certain islands off the south coasts of Burmah, flat rocks covered with oysters are laid bare at low tide. A species of Monkey (Macacus cynomolgus) has been noticed to furnish himself with a stone, and knock the oysters open, always breaking the hinge-end first, and then pulling out the mollusc with his fingers.[140]
The walrus is said to support himself almost entirely on two species of Mya (truncata and arenaria), digging them out of the sand, in which they live buried at a depth of about 1½ feet, with his powerful tusks. Whales swallow enormous numbers of pelagic molluscs (Clio, Limacina), which are at times so abundant in the Arctic seas, as to colour the surface for miles. Many of the larger Cetacea subsist in great part on Cephalopoda; as many as 18 lbs. of beaks of Teuthidae have been taken from the stomach of a single Hyperoodon.
Fish are remarkably partial to Mollusca of various kinds. The cat-fish (Chimaera) devours Pectunculus and Cyprina, crushing the stout shells with its powerful jaws, while flounders and soles content themselves with the smaller Tellina and Syndosmya which they swallow whole. As many as from 30 to 40 specimens of Buccinum undatum have been taken from the stomach of a single cod, and the same ‘habitat’ has been recorded for some of the rarer whelks, e.g. Bucc. humphreysianum, Fusus fenestratus, the latter also occurring as the food of the haddock and the red gurnard. No less than 35,000 Turtonia minuta have been found in the stomach of a single mullet.[60] Nudibranchs are no doubt dainty morsels for fish, and hence have developed, in many cases, special faculties for concealment, or, if distasteful, special means of remaining conspicuous (see pp. 71–74).
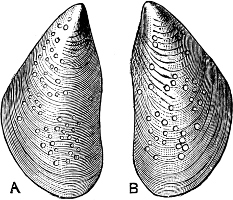
Fig. 22.—Two valves of Mytilus edulis L., representing diagrammatically the approximate position of the holes bored by Purpura in about 100 specimens of Mytilus, gathered at Newquay, Cornwall.
Besides the dangers to which they are exposed from other enemies, many of the weaker forms of Mollusca fall a prey to their own brethren. Nassa and Murex on this side of the Atlantic, and Urosalpinx on the other, are the determined foes of the oyster. Purpura lapillus prefers Mytilus edulis to any other food, piercing the shell in about two days’ time by its powerful radula, which it appears to employ somewhat in gimlet fashion. If Mytilus cannot be procured, it will eat Littorina or Trochus, but its attempts on the hard shell of Patella are generally failures. The statement which is sometimes made, that the Purpura makes its hole over the vital parts of the Mytilus, appears, according to the evidence embodied in the annexed figure, to be without foundation. The fact is that a hole in any part of its shell is fatal to the Mytilus, since the long proboscis of the Purpura, having once made an entrance, can reach from one end of the shell to the other. The branchiae are first attacked, the adductor muscles and edges of the mantle last. Natica and Nassa pierce in a similar way the shells of Mactra, Tellina, Donax, and Venus. Murex fortispina is furnished with a powerful tooth at the lower part of its outer lip. At Nouméa, in New Caledonia, its favourite food is Arca pilosa, which lives half buried in coral refuse. The Murex has been seen to drag the Arca from its place of concealment, and insert the tooth between the valves, so as to prevent their closing, upon which it was enabled to devour its prey at leisure.[141]
The carnivorous land Mollusca, with the exception of Testacella, appear to feed by preference upon other snails (pp. 54, 55).
[61]
Parasitic Worms, Mites, etc.—A considerable number of the Trematode worms pass one or more of the stages in the cycle of their development within the bodies of Mollusca, attaining to the more perfect or sexual form on reaching the interior of some vertebrate. Thus Distoma endolabum Duj. finds its first intermediate host in Limnaea stagnalis and L. ovata, its second in L. stagnalis, or in one of the fresh-water shrimps (Gammarus pulex), or in the larvae of one of the Phryganeidae (Limnophilus rhombicus), attaining to the sexual form in the common frog. Distoma ascidia v. Ben. passes firstly through Limnaea stagnalis or Planorbis corneus, secondly through certain flies and gnats (Ephemera, Perla, Chironomus), and finally arrives within certain species of bats. Distoma nodulosum Zed. inhabits firstly Paludina impura, secondly certain fishes (Cyprinus Acerina), and lastly the common perch. The sporocyst of Distoma macrostomum inhabits Succinea putris, pushing itself up into the tentacles, which become unnaturally distended (Fig. 23). While in this situation it is swallowed by various birds, such as the thrush, wagtail, and blackbird, which are partial to Succinea, and thus obtains lodgment in their bodies. Amphistoma subclavatum spends an early stage in Planorbis contortus, after which it becomes encysted on the skin of a frog. When the frog sheds its skin, it swallows it, and with it the Amphistoma, which thus becomes established in the frog’s stomach.[142]
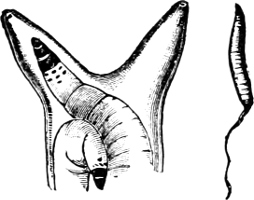
Fig. 23.—A Trematode worm (Leucochloridium paradoxum Car.) parasitic in the tentacles of Succinea putris L. × 20 (after Baudon).
The common liver-fluke, which in the winter of 1879–1880 cost Great Britain the lives of no less than three million sheep, is perhaps the best known of these remarkable parasitic forms of life. Its history shows us, in one important particular, how essential it is for the creature to meet, at certain stages of its existence, with the exact host to which it is accustomed. Unless the newly-hatched embryo finds a Limnaea truncatula within about eight hours it becomes exhausted, sinks, and dies. It has been tried with all the other common pond and river Mollusca, with Limnaea peregra, palustris, auricularia, stagnalis, with Planorbis marginatus, carinatus, vortex, and spirorbis, with Physa fontinalis, Bithynia tentaculata, Paludina vivipara, as[62] well as with Succinea putris, Limax agrestis and maximus, Arion ater and hortensis. Not one of them would it touch, except occasionally very young specimens of L. peregra, and in these its development was arrested at an early stage. But on touching a L. truncatula the embryo seems to know at once that it has got what it wants, and sets to work immediately to bore its way into the tissues of its involuntary host, making by preference for the branchial chamber; those which enter the foot or other outlying parts of the Limnaea proceed no farther.[143]
Many similar cases occur, in which littoral Mollusca, such as Littorina and Buccinum, form the intermediate host to a worm which eventually arrives within some sea-bird.
Certain Nematode worms (Rhabditis) are known to inhabit the intestine of Arion, and the salivary glands of Limax agrestis. Diptera habitually lay their eggs within the eggs of Helix and Limax. Many species of mite (Acarina) infest land Pulmonata. No adult Limax maximus is without at least one specimen of Philodromus (?) limacum, and the same, or an allied species, appears to occur on the larger of our Helices, retiring upon occasion into the pulmonary chamber.
Several of the Crustacea live associated with certain molluscs. Pinnotheres lives within the shell of Pinna, Ostrea, Astarte, Pectunculus, and others. Apparently the females alone reside within the shell of their host, while the males seize favourable opportunities to visit them there. A specimen of the great pearl-oyster (Meleagrina margaritifera) was recently observed which contained a male Pinnotheres encysted in nacre. It was suggested that he had intruded at an unfortunate time, when no female of his kind happened to be in, and that, having penetrated too far beneath the mantle in the ardour of his search, was made prisoner before he could escape.[144] Ostracotheres Tridacnae lives in the branchiae of the great Tridacna. A little brachyurous crustacean inhabits the raft of Ianthina, and assumes the brilliant blue colour of the mollusc.
As a rule, among the Mollusca, the shell forms a passive mode of resistance to the attacks of enemies. Bivalves are[63] enabled, by closing their valves, to baffle the assault of their smaller foes, and the operculum of univalves, both marine and land, serves a similar purpose. Many land Mollusca, especially Helix and Pupa, as well as a number of Auriculidae, have the inside of the aperture beset with teeth, which are sometimes so numerous and so large that it is puzzling to understand how the animal can ever come out of its shell, or, having come out, can ever draw itself back again. Several striking cases of these toothed apertures are given in Fig. 24. Whatever may be the origin of these teeth, there can be little doubt that their extreme development must have a protective result in opposing a barrier to the entrance, predatory or simply inquisitive, of beetles and other insects. Sometimes, it will be noticed (G), the aperture itself is fairly simple, but a formidable array of obstacles is encountered a little way in. It is possible that the froth emitted by many land snails has a similar effect in involving an irritating intruder in a mass of sticky slime. The mucus of slugs and snails, on the other hand, is more probably, besides its use in facilitating locomotion, a contrivance for checking evaporation, by surrounding the exposed parts of their bodies with a viscid medium.
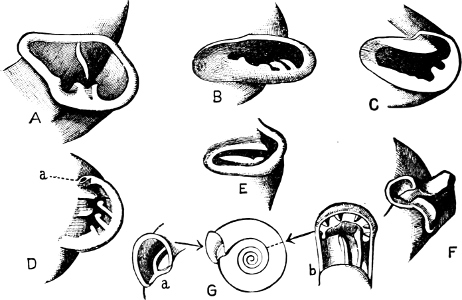
Fig. 24.—Illustrating the elaborate arrangement of teeth in the aperture of some land Pulmonata. A. Helix (Labyrinthus) bifurcata Desh., Equador. B. H. (Pleurodonta) picturata Ad., Jamaica. C. H. (Dentellaria) nux denticulata Chem., Demerara. D. Anostoma carinatum Pfr., Brazil; a, tube communicating with interior of shell. E. H. (Stenotrema) stenotrema Fér., Tennessee, × 3/2. F. H. (Polygyra) auriculata Say, Florida, × 3/2. G. H. (Plectopylis) refuga Gld., Tenasserim (a and b × 2).
Some species of Lima shelter themselves in a nest constructed of all kinds of marine refuse, held together by byssiferous threads.[64] Modiola adriatica, M. barbata, and sometimes M. modiolus conceal themselves in a similar way. Gastrochaena frequently encloses itself in a sort of half cocoon of cement-like material. The singular genus Xenophora protects itself from observation by gluing stones, shells, and various débris to the upper side of its whorls (Fig. 25). Sometimes the selection is made with remarkable care; the Challenger, for instance, obtained a specimen which had decorated its body whorl exclusively with long and pointed shells (Fig. 26).

Fig. 25.—Xenophora (Phorus) conchyliophora Born., concealed by the stones which it glues to the upper surface of its shell. (From a British Museum specimen.)
Fig. 26.—Xenophora (Phorus) pallidula Reeve. A mollusc which escapes detection by covering itself with dead shells of other species. (From a Challenger specimen in the British Museum, × ½.)
The formidable spines with which the shells, e.g. of the Murex family, are furnished must contribute greatly to their protection against fishes, and other predatory animals. Murex tenuispina, for instance (see chap. ix.), would prove as dangerous a morsel in the mouth of a fish as a hedgehog in that of a dog. Whether the singular tooth in the outer lip of Leucozonia (see chap. xiv.), a feature which is repeated, to a less marked extent, in Monoceros and several of the West Coast muricoids, is developed for defensive purposes, cannot at present be decided.
The Strombidae possess the power of executing long leaps, which they doubtless employ to escape from their foes. In their case alone this power is combined with singular quickness of vision. On one occasion Mr. Cuming, the celebrated collector, lost a beautiful specimen of Terebellum, by the animal suddenly[65] leaping into the water, as he was holding and admiring it in his hand. Miss Saul has informed me that the first living specimen of Trigonia that was ever obtained was lost in a similar way. It was dredged by Mr. Stutchbury in Sydney Harbour, and placed on the thwart of a small boat. He had just remarked to a companion that it must be a Trigonia, and his companion had laughed at the idea, reminding him that all known Trigonia were fossil, when the shell in question baffled their efforts to discover its generic position by suddenly leaping into the sea, and it was three months before Mr. Stutchbury succeeded in obtaining another.
Some genera possess more than merely passive means of defence. Many Cephalopoda emit a cloud of inky fluid, which is of a somewhat viscous nature, and perhaps, besides being a means of covering retreat, serves to entangle or impede the pursuer. The formidable suckers and hooks possessed by many genera in this Order are most dangerous weapons, both for offence and defence. Aplysia, when irritated, ejects a purple fluid which used to be considered dangerously venomous. Many of the Aeolididae, including our own common Aeolis papillosa, possess stinging cells at the end of their dorsal papillae, the effect of which is probably to render them exceedingly distasteful to fish.
The common Vitrina pellucida has a curious habit which in all probability serves for a defence against birds in the winter. When crawling on the edge of a stone or twig it has the power of suddenly jerking its ‘tail,’ so as to throw itself on the ground, where it is probably lost to sight among decaying leaves. At other times it rolls away a few inches and repeats the jump. It also possesses the power of attaching to itself bits of leaves or soil, which entirely cover and conceal both shell and animal.[145] The property of parting with the tail altogether, a remarkable form of self-defence, has already been noticed on p. 44.
The poisonous nature of the bite of certain species of Conus is well authenticated. Surgeon Hinde, R.N., saw[146] a native on the I. of Matupi, New Britain, who had been bitten by a Conus geographus, and who had at once cut small incisions with a sharp stone all over his arm and shoulder. The blood flowed freely,[66] and the native explained that had he not taken these precautions he would have died. Instances have been recorded of poisonous wounds being inflicted by the bite of Conus aulicus, C. textile, and C. tulipa. According to Mr. J. Macgillivray[147] C. textile at Aneitum (S. Pacific) is called intrag, and the natives say it spits the poison upon them from several inches off! Two cases of bites from C. textile occurred to this gentleman’s notice, one of which terminated fatally by gangrene. Sir Edward Belcher, when in command of the Samarang, was bitten[148] by a Conus aulicus at a little island off Ternate in the Moluccas. As he took the creature out of the water, it suddenly exserted its proboscis and inflicted a wound, causing a sensation similar to that produced by the burning of phosphorus under the skin. The wound was a small, deep, triangular mark, succeeded by a watery vesicle. The natives of New Guinea have a wholesome dread of the bite of Cones. Mr. C. Hedley relates[149] that while collecting on a coral reef he once rolled over a boulder and exposed a living C. textile. Before he could pick it up, one of the natives hastily snatched it away, and explained, with vivid gesticulations, its hurtful qualities. On no account would he permit Mr. Hedley to touch it, but insisted on himself placing it in the bottle of spirits.
Fig. 27.—A tooth from the radula of Conus imperialis L., × 50, showing barb and poison duct.
Cases of Mimicry, or protective resemblance, when a species otherwise defenceless adopts the outward appearance of a better protected species, are rare among the Mollusca. Karl Semper[150] mentions an interesting case of the mimicry of Helicarion tigrinus by Xesta Cumingii, in the Philippines. It appears that all[67] species of Helicarion possess the singular property of shaking off the ‘tail’ or hinder part of the foot, when seized or irritated. Specimens captured by collectors, Hel. tigrinus amongst them, have succeeded in escaping from the hand, and concealing themselves, by a sort of convulsive leap, among the dry leaves on the ground. This power of self-amputation must be of great value to Helicarion, not only as enabling it to escape from the clutch of its enemies, but also as tending to discourage them from attempting to capture it at all. Now the genus Xesta is, in anatomy, very far removed from Helicarion, and the majority of the species are also, as far as the shell is concerned, equally distinct. Xesta Cumingii, however, has, according to Semper, assumed the appearance of a Helicarion, the thin shell, the long tail, and the mantle lobes reflected over the shell; but it has not the power of parting with its tail at short notice. It lives associated with Helicarion, and so close is the resemblance between them that, until Semper pointed out its true position, it had always been classified as a member of that group.
In the same passage Semper draws attention to two other cases of apparent mimicry. The first is another species of Xesta (mindanaensis) which closely resembles a species of Rhysota (Antonii), a genus not indeed so far removed from Xesta as Helicarion, but, as far as the shell is concerned, well distinguished from it. In this case, however, there is no obvious advantage gained by the resemblance, since Rhysota as compared with Xesta is not known to possess any definite point of superiority which it would be worth while to counterfeit. A second case of resemblance between certain species of the genus Chloraea and the characteristic Philippine group Cochlostyla will not hold good as affording evidence of mimicry, for Chloraea is now recognised as a sub-genus of Cochlostyla.
The Mollusca are not much mimicked by creatures of different organisation. This appears at first sight strange, since it might have been thought that the strong defensive house of a snail was worth imitating. Still it is probably not easy for creatures bilaterally symmetrical to curl themselves up into an elevated spiral for any length of time. One or two instances, however, may be mentioned. The larva of a moth belonging to the Psychidae, and occurring in France, Germany, the Tyrol, and Syria, coils itself up into a sinistral spiral of three whorls,[68] and is aptly named Psyche helix, a kindred species from Italy being known as Ps. planorbis.
An insect larva (Cochlophora valvata) from E. Africa is said to resemble a Valvata or young Cyclostoma. In this case the spiral is indifferently dextral or sinistral, the ‘shell’ being formed of masticated vegetable matter, united together by threads spun by the larva. Certain larvae of the Phryganeidae (“Caddis-worms”) enclose themselves in houses which more or less resemble a spiral shell, and have in some cases actually been described as molluscan; such species, some of which belong to Helicopsyche, have been noticed in S. Europe, Ceylon, Further India, China, Tasmania, New Zealand, Tennessee, Mexico, Central America, Venezuela, Brazil, and Argentina, and all[151] possess a dextral ‘shell.’ In all these cases ‘mimicry’ is probably not so much to be thought of as the practical advantages which accrue to the animal in question from the spiral form, which gives it greater strength to resist external blows, and enables it to occupy, during a very defenceless portion of its existence, a very small amount of space.
The larva of some species of the Syrphidae (Diptera) fixes itself on the under side of stones in the Tyrol, and closely resembles a small slug. The naturalist Von Spix, in 1825, described to the Bavarian Academy as a new genus of land Mollusca a somewhat similar larval form found in decaying wood on the banks of a German lake.[152] Simroth mentions[153] a curious case as occurring near Grimma. The caterpillars of certain Microlepidoptera occur on slabs of porphyry, associated with a species of Clausilia. Besides being of the same colour as the Clausiliae, the caterpillars have actually developed cross lines on the back, i.e. on the side turned away from the rock, in imitation of the suture of the mollusc.
It has been suggested[154] that there is mimicry between Aeolis papillosa (a common British nudibranch) and Sagartia troglodytes (an Actinian), and also between another species of Sagartia and Aeolidiella Alderi. The facts observed are not sufficient to warrant a decided opinion, but it seems more probable[69] that the Actinian mimics the nudibranch than vice versâ, since Aeolis is known to be unpalatable to fishes.
Fig. 28.—A, Strombus mauritianus Lam., which mimics Conus in shape. B, Conus janus Hwass, Mauritius.
Certain species of Strombus (mauritianus L., luhuanus L.) show a remarkable similarity in the shape of the shell to that of Conus, so much so, that a tiro would be sure to mistake them, at first sight, for Cones. In the case of S. luhuanus at least, this similarity is increased by the possession of a remarkably stout brown epidermis. Now Conus is a flesh-eating genus, armed with very powerful teeth which are capable of inflicting even on man a poisonous and sometimes fatal wound (see p. 66). Strombus, on the other hand, is probably frugivorous, and is furnished with weak and inoffensive teeth. It is possible that this resemblance is a case of ‘mimicry.’ It is quite conceivable that powerful fishes which would swallow a Strombus whole and not suffer for it, might acquire a distaste for a Cone, which was capable of lacerating their insides after being swallowed. And therefore the more like a Cone the Strombus became, the better chance it would have of being passed over as an ineligible article of food.
Protective coloration is not uncommon among the Mollusca. Littorina obtusata is habitually found, on our own coasts, on Fucus vesiculosus, the air-bladders of which it closely resembles in colour and shape. Littorina pagodus, a large and showy species, resembles so closely the spongy crumbling rocks of Timor, on which it lives, that it can hardly be discerned a pace off. Helcion pellucidum, the common British ‘blue limpet,’ lives, when young, almost exclusively on the iridescent leaves of the great Laminariae, with the hues of which its own conspicuous blue lines harmonise exactly. In mature life, when the Helcion invariably transfers its place of abode to the lower parts of the stalk and finally to the root of the Laminaria, which are quite destitute of iridescence, these blue lines disappear or become much less marked.
The specimens of Purpura lapillus which occur at Newquay[70] in Cornwall are banded with rings of colour, especially with black and white, in a more varied and striking way than any other specimens that have ever occurred to my notice. I am inclined to refer this peculiarity to a tendency towards protective coloration, since the rocks on which the Purpura occurs are often banded with veins of white and colour, and variegated to a very marked extent.
Ovula varies the colour of its shell from yellow to red, to match the colour of the Gorgonia on which it lives. The same is the case with Pedicularia, which occurs on red and yellow coral.
Helix desertorum, by its gray-brown colour, harmonises well with the prevailing tint of the desert sands, among which it finds a home. Benson observes that the gaudy H. haemastoma, which lives on the trunks of palm-trees in Ceylon, daubs its shell with its excrement. Our own Buliminus obscurus, which lives principally on the trunks of smooth-barked trees, daubs its shell with mud, and must often escape the observation of its enemies by its striking resemblance to the little knots on the bark, especially of beech trees, its favourite haunt. Some species of Microphysa, from the West Indies, habitually encrust their shells with dirt, and the same peculiarity in Vitrina has already been mentioned. Ariophanta Dohertyi Aldr., a recent discovery from Sumatra, is of a green colour, with a singularly delicate epidermis; it is arboreal in its habits, and is almost invisible amongst the foliage.[155] Many of our own slugs, according to Scharff, are coloured protectively according to their surroundings. A claret-coloured variety of Arion ater occurred to this observer only in pine woods, where it harmonised with the general colouring of the ground and the pine-needles, while young winter forms of the same species choose for hiding-places the yellow fallen leaves, whose colour they closely resemble. Limax marginatus (= arborum Bouch.) haunts tree trunks, and may easily be mistaken for a piece of bark; Amalia carinata lives on and under the ground, and in colour resembles the mould; Arion intermedius feeds almost exclusively on fungi, to which its colour, which is white, gray, or light yellow, tends to approximate it closely; Geomalacus maculosus conceals itself[71] by its striking resemblance to the lichens which grow on the surface of rocks, and actually presumes on this resemblance so much as to expose itself, contrary to the usual custom of its congeners, to the full light of the afternoon sun.[156]
Several views have been advanced with regard to the dorsal papillae, or cerata, in the Nudibranchs. Professor W. A. Herdman, who has examined a considerable number of our own British species, in which these processes occur, is of opinion[157] that they are of two quite distinct kinds. In the first place, they may contain large offshoots, or diverticula, of the liver, and thus be directly concerned in the work of digestion. This is the case with Aeolis and Doto. In the second place, they may be simply lobes on the skin, with no connexion with the liver, and no special function to perform. This is the case with Tritonia, Ancula, and Dendronotus.
Professor Herdman is of opinion that although the cerata may in all cases aid in respiration to a certain extent, yet that extent is so small as to be left out of consideration altogether. He regards the cerata in both the two classes mentioned above as “of primary importance in giving to the animals, by their varied shapes and colours, appearances which are in some cases protective, and in others conspicuous and warning.”
Thus, for instance, Tritonia plebeia, which is fairly abundant at Puffin and Hilbre Is., appears always to be found creeping on the colonies of a particular polyp, Alcyonium digitatum, and nowhere else. The specimens in each colony of the polyp differ noticeably both in the matter of colour, and of size, and of varied degrees of expansion. The Tritonia differs also, being marked in varied tints of yellow, brown, blue, gray, black, and opaque white, in such a way as to harmonise with the varied colours of the Alcyonium upon which it lives. The cerata on the back of the Tritonia contribute to this general resemblance. They are placed just at the right distance apart, and are just the right size and colour, to resemble the crown of tentacles on the half-expanded polyp.
Similarly, Doto coronata, which, when examined by itself, is a very conspicuous animal, with showy, bright-coloured cerata, is found by Professor Herdman to haunt no other situations but[72] the under side of stones and overhanging ledges of rock which are colonised by a hydroid, known as Clava multicornis. The Doto is masked by the tentacles and clusters of sporosacs on the zoophyte, with whose colouring and size its own cerata singularly correspond. A similar and even more deceptive correspondence with environment was noticed in the case of the very conspicuous Dendronotus arborescens.
In these cases, the colouring and general shape of the cerata are protective, i.e. they match their surroundings in such a way as to enable the animal, in all probability, to escape the observation of its enemies. According to Professor Herdman, however, the brilliant and showy coloration of the cerata of Aeolis is not protective but ‘warning.’ Aeolis does not hide itself away as if shunning observation, like Doto, Tritonia, and Dendronotus; on the contrary, it seems perfectly fearless and indifferent to being noticed. Its cerata are provided with sting-cells, like those of Coelenterata, at their tips, and its very conspicuousness is a warning to its enemies that they had better not try to attack it, just as the showy white tail of the skunk acts as a sort of danger-signal to its own particular foes. It is important for the Aeolis, not merely to be an unpalatable nettle in animal shape, but also to be conspicuous enough to prevent its being experimented upon as an article of food, in mistake for something less nasty.
Professor Herdman subsequently conducted some experiments[158] with fishes, with the view of testing his theory that the shapes and colours of Nudibranchs serve the purpose either of protection or warning, and bear direct relation to the creature’s edibility. These experiments, on the whole, distinctly tended to confirm the theory. Aeolis was evidently very nasty, and probably stung the mouths of the fishes who tried it. For the complete success of the theory, they ought to have let it severely alone, but the fish were evidently accustomed to make a dash at anything that was dropped into their tank. Another conspicuous mollusc, Ancula cristata, was introduced, Professor Herdman and his collaborator each commencing operations by eating a live specimen themselves. They found the taste pleasant, distinctly like that of an oyster. The fish, however,[73] when the experiments were conducted under conditions which made the scene as much like ‘real life’ as possible, did not agree with Professor Herdman. The Ancula crawled over various parts of the tank for several days untouched by the fish, who sometimes went close to them and looked at them, but never attempted to taste them. Experiments with species whose colours were protective, such as Dendronotus, were also conducted, and the decided edibility of these species was established, the fish competing eagerly for them, and tearing them rapidly to pieces.
Mr. W. Garstang, of the Plymouth Laboratory of the Marine Biological Association, confirms[159] Professor Herdman’s views as to the shape and colour of Opisthobranchs. Pleurobranchus membranaceus is known to secrete, on the surface of the body, an acid which reddens blue litmus paper. It is, therefore, no doubt distasteful to fish, which all abominate the taste of acids, and is conspicuously marked with red-brown and yellowish ‘warning’ colours. Haminea and Philine, on the other hand, are good to eat, and consequently possess ‘protective’ coloration. Runcina Hancocki, which is of a brown colour, crawls over brown mud and weeds, but avoids green weeds, on whose surface it would appear conspicuous. Elysia viridis varies its colour according to its habitat, being green when on green weeds, and dark olive, brown, or reddish brown, on pools among tufts of littoral algae. Green specimens of Hermaea dendritica were kept in captivity, and placed in a dish with green and red sea-weeds. They were never observed crawling upon the red weed, upon which they would have been very conspicuous. Archidoris flammea occurred on bright red sponges, to which its colour was so closely assimilated that Mr. Garstang at first quite overlooked it. Goniodoris castanea was found under stones, feeding on compound Ascidians (Botryllus), which it sufficiently resembled to be very inconspicuous in that position.
Again, Jorunna Johnstoni lives[160] upon stones on our southern coast, associated with a certain sponge (Halichondria sp.), which it resembles so closely in outline, in colour, in character of surface, and in its projecting plumes, as to make it very difficult[74] even for the careful observer to distinguish the one from the other. And, since fishes, are known to be distinctly averse to sponges of any kind as an article of food, this resemblance must be decidedly to the advantage of the Jorunna. Another Nudibranch (Calma glaucoides A. and H.) imitates the ova of certain fishes, on which it feeds. Its elongated and depressed form of body, transparent integuments, and silvery gray papillae combine to give it a strong resemblance to the spawn of the fish, which is deposited on stones, the roots of Laminaria, etc.[161]
The common Lamellaria perspicua appears to possess the power of protectively assimilating its colour, markings, etc., to the Ascidians on which it lives. A recent case, occurring off the Isle of Man, is thus described by Professor Herdman.[162] “The mollusc was on a colony of Leptoclinum maculatum, in which it had eaten a large hole. It lay in this cavity so as to be flush with the general surface; and its dorsal integument was not only whitish with small darker marks which exactly reproduced the appearance of the Leptoclinum surface with the ascidiozooids scattered over it, but there were also two larger elliptical clear marks which looked like the large common cloacal apertures of the Ascidian colony.... Presumably the Lamellaria escapes the observation of its enemies through being mistaken for part of the Leptoclinum colony; and the Leptoclinum, being crowded like a sponge with minute sharp-pointed spicules, is, I suppose, avoided as inedible (if not actually noxious through some peculiar smell or taste) by carnivorous animals which might devour such things as the soft unprotected mollusc.”
Various grades of parasitism occur among the Mollusca, from the true parasite, living and nourishing itself on the tissues and secretions of its host, to simple cases of commensalism. Some authors have divided these forms into endo- and ectoparasites, according as they live inside or outside of their host.[75] Such a division, however, cannot be rigidly carried out, for certain forms are indifferently endo- and ecto-parasitical, while others are ecto-parasitic in the young form, and become endo-parasitic in the adult. It will be convenient, therefore, simply to group the different forms according to the home on which they find a lodgment.
Fig. 29.—Magilus antiquus L.: A, the adult, imbedded in coral, which has been broken away to show the tube; B, the young (free) form.
On Sponges.—Vulsella and Crenatula almost invariably occur in large masses of irregular shape, boring into sponges. They are especially abundant on Porifera from the Red Sea. Corals form a favourite home of many species, amongst which are several forms of Coralliophila, Rhizochilus, Leptoconchus, and Sistrum. Rhizochilus is a very singular creature, inhabiting branching corals. When adult, it forms irregular shelly extensions of both the inner and outer lips, which adhere to the shafts of the coral, or to the surface of neighbouring shells; at length the aperture becomes completely closed with the exception of the siphonal tube, which becomes long, and consists of the same shelly material. The common Magilus (Fig. 29), from the Red Sea and Indian Ocean, in the young form is shaped like a small Buccinum. As the coral (Meandrina) to which it attaches itself grows, the Magilus develops at the mouth a long calcareous tube, the aperture of which keeps pace with the growth of the coral, and prevents the mollusc from being entombed. The animal lives at the free, or outer, end of the tube, and is thus continually shifting its position, while the space it abandons becomes completely closed by a mass of solid calcareous matter. Certain species of Ovula inhabit Gorgonia, assuming the colour, yellow or red, of their host, and, in certain cases, developing, probably for prehensile purposes, a pointed extension of the two extremities of the shell. Pedicularia, a form akin to Cypraea, but with a more patulous mouth, inhabits the common Corallium rubrum of the Mediterranean, and[76] another species has been noticed by Graeffe[163] on Melithaea ochracea in Fiji.
On Echinodermata.—(a) Crinoidea. Stylina comatulicola lives on Comatula mediterranea, fixed to the outer skin, which it penetrates by a very long proboscis; the shell is quite transparent.[164] A curious case of a fossil parasite has been noticed by Roberts.[165] A Calyptrea-shaped shell named Platyceras always occurred on the ventral side of a crinoid, encompassed by the arms. For some time this was thought to afford conclusive proof of the rapacity and carnivorous habits of the echinoderm, which had died in the act of seizing its prey. Subsequent investigations, however, showed that in all the cases noticed (about 150) the Platyceras covered the anal opening of the crinoid in such a way that the mouth of the mollusc must have been directly over the orifice of the anus. (b) Asteroidea. The comparatively soft texture of the skin of the starfishes renders them a favourite home of various parasites. The brothers Sarasin noticed[166] a species of Stilifer encysted on the rays of Linckia multiformis. Each shell was enveloped up to the apex, which just projected from a hole at the top of the cyst. The proboscis was long, and at its base was a kind of false mantle, which appeared to possess a pumping action. On the under side of the rays of the same starfish occurred a capuliform mollusc (Thyca ectoconcha), furnished with a muscular plate, whose cuticular surface was indented in such a way as to grip the skin of the Linckia. This plate was furnished with a hole, through which the pharynx projected into the texture of the starfish, acting as a proboscis and apparently furnished with a kind of pumping or sucking action. Adams and Reeve[167] describe Pileopsis astericola as living ‘on the tubercle of a starfish,’ and Stilifer astericola, from the coast of Borneo, as ‘living in the body of a starfish.’ In the British Museum there is a specimen of Pileopsis crystallina ‘in situ’ on the ray of a starfish, (c) On the brittle starfishes (Ophiuroidea) occur several species of Stiliferina. (d) Echinoidea. Various species of Stilifer occur on the ventral spines of[77] echinoids, where they probably subsist on the excreta, and are sometimes found imbedded in the spines themselves. St. Turtoni occurs on the British coasts on several species of Echinus, and Montacuta substriata frequents Spatangus purpureus and certain species of Echinocardium, Cidaris, and Brissus. Lepton parasiticum has been described from Kerguelen I. on a Hemiaster, and a new genus, Robillardia, has recently been established[168] for a Hyalinia-shaped shell, parasitic on an Echinus from Mauritius. (e) Holothurioidea. The ‘sea-cucumbers’ afford lodgment to a variety of curious forms, some of which have experienced such modifications that their generic position is by no means established. Entoconcha occurs fixed by its buccal end to the blood-vessels of certain Synapta in the Mediterranean and the Philippines. Entocolax has been dredged from 180 fath. in Behring’s Straits, attached by its head to certain anterior muscles of a Myriotrochus.[169] A curious case of parasitism is described by Voeltzkow[170] as occurring on a Synapta found between tide-marks on the I. of Zanzibar. In the oesophagus of the Synapta was found a small bivalve (Entovalva), the animal of which was very large for its shell, and almost entirely enveloped the valves by its mantle. As many as five specimens occurred on a single Synapta. In the gut of the same Holothurian lived a small univalve, not creeping freely, but fixed to a portion of the stomach wall by a very long proboscis which pierced through it into the body cavity. This proboscis was nearly three times as long as the animal, and the forward portion of it was set with sharp thorns, no doubt in order to enable it to retain its hold and resist evacuation. Various species of Eulima have been noticed in every part of the world, from Norway to the Philippines, both inside and outside Holothurians.[171] Stilifer also occurs on this section of Echinoderms.[172]
On Annelida.—Cochliolepis parasiticus has been noticed under the scales of Acoetes lupina (a kind of ‘sea-mouse’) in Charleston Harbour.[173]
On Crustacea.—A mussel, ⅜ in. long, has been found[174] living[78] under the carapace of the common shore-crab (Carcinus maenas), and one case has been noticed[175] where two mussels, one of several months’ growth, the other smaller, well secured by their byssi, were found under the abdomen of the same species, in such a position as to force the appendages apart and askew. These, however, are not so much cases of parasitism as of involuntary habitat, the mussel no doubt having become involved in the branchiae and the abdomen of the crab in the larval form.
On Mollusca.—A species of Odostomia (pallida Mont.) is found on our own coasts on the ‘ears’ of Pecten maximus, and also[176] on the operculum of Turritella communis. Another species (O. rissoides) frequently occurs in hiding under beds of mussels, but it is not clear whether the habitat is due to parasitism, or simply to the fact that the mass of mussels, knitted together and to the rock by the byssi, affords the Odostomia a safe lurking-place. At Panama the present writer found Crepidula (2 sp.) plentiful on the opercula of the great Strombus galea and of Cerithium irroratum. In each case the parasite exactly fitted the size of the operculum, and had assumed its colour, dark brown or chestnut. Amalthea is very commonly found on Conus, Turbo, and other large shells from the South Pacific, but this is probably not a case of parasitism, but simply of convenience of habitat, just as young oysters are frequently seen on the carapace and even on the legs of large crabs.
Fig. 30.—Crepidula onyx Sowb., parasitic on the operculum of Strombus galeatus Swains., Panama.
On Tunicata.—Lamellaria deposits its eggs and lives on an Ascidian (Leptoclinum), and the common Modiolaria marmorata lives in colonies imbedded in the test of Ascidia mentula and other simple Ascidians.
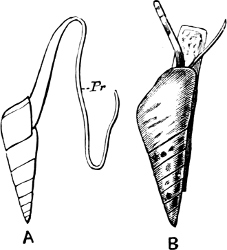
Fig. 31.—Two species of Eulima: A is sessile on the skin of a Holothurian, through which it plunges its sucking proboscis (Pr); B creeps freely in the stomach of a Holothurian. (After K. Semper.)
Special points of interest with regard to parasitic Mollusca relate to (1) Colour. This is in most cases absent, the shell being of a uniform hyaline or milky white. This may be due, in the case of the endo-parasitic forms, to absence of light, and possibly, in those living outside their host, to some deficiency in the nutritive material. A colourless shell is not necessarily protective,[79] for though a transparent shell might evade detection, a milk-white hue would probably be conspicuous. (2) Modifications of structure. These are in many cases considerable. Entoconcha and Entocolax have no respiratory or circulatory organs, and no known nervous system; Thyca and certain Stilifer possess a curious suctorial apparatus; the foot in many cases has aborted, since the necessity for locomotion is reduced to a minimum, and its place is supplied by an enormous development of the proboscis, which enables the creature to provide itself with nutriment without shifting its position. K. Semper notices a case where a Eulima, whose habitat is the stomach of a Holothurian, retains the foot unmodified, while a species occurring on the outer skin, but provided with a long proboscis, has lost its foot altogether.[177] Special provision for holding on is noticed in certain cases, reminding us of similar provision in human parasites. Eyes are frequently, but not always wanting, even in endo-parasitic forms. A specially interesting modification of structure occurs in (3) the Radula or ribbon-shaped arrangement of the teeth. In most cases of parasitism (Eulima, Stilifer, Odostomia, Entoconcha, Entocolax, Magilus, Coralliophila, Leptoconcha) it is absent altogether. In Ovula and Pedicularia, genera which are in all other respects closely allied to Cypraea, the radula exhibits marked differences from the typical radula of the Cypraeidae. The formula (3·1·3) remains the same, but the laterals are greatly produced and become fimbriated, sometimes at the extremity only, sometimes along the whole length. A very similar modification occurs in the radula of Sistrum spectrum Reeve, a species which is known to live parasitically on one of the branching corals. Here the laterals differ from those of the typical Purpuridae in being very long and curved at the extremity. The general effect of these modifications appears to be the production of a radula rather of the type of the vegetable-feeding Trochidae, which may perhaps be regarded as[80] a link in the chain of gradually-degraded forms which eventually terminate in the absence of the organ altogether. The softer the food, the less necessity there is for strong teeth to tear it; the teeth either become smaller and more numerous, or else longer and more slender, and eventually pass away altogether. It is curious, however, that the same modified form of radula should appear in species of Ovula (e.g. ovum) and that the same absence of radula should occur in species of Eulima (e.g. polita) known to be not parasitic. This fact perhaps points back to a time when the ancestral forms of each group are parasitic and whose radulae were modified or wanting, the modification or absence of that organ being continued in some of their non-parasitical descendants.
Mollusca are concerned in several interesting cases of commensalism, or the habitual association of two organisms, as distinguished from parasitism, where one form preys more or less upon the other.
Mr. J. T. Marshall has given[178] an interesting account of the association of Montacuta ferruginosa with Echinocardium cordatum. The Echinoderm lives in muddy sand in Torbay, at a depth of about 6 inches, and the Montacuta lives in a burrow leading from its ventral end and running irregularly in a sloping direction for 3 or 4 inches, the burrow, which is made by a current from the Echinoderm, being almost exactly the width of the Montacuta. The Montacuta were always arranged in the burrows in order of size, the largest being close to the Echinoderm, and the smallest of a string of about six at the other end of the burrow. In another part of S. Devon, where the sand was soft and sloppy, the Echinocardia rise to the surface and travel along the sand; in this case the Montacuta were attached to their host by means of a byssus, and were dragged along as it travelled.
The Rev. Dr. Norman has noted[179] a somewhat similar habitat for Lepton squamosum. This rare little British species was found at Salcombe, living in the burrows of Gebia stellata, in all probability feeding upon the secretions from the body of the[81] crustacean. Dr. Norman suggests that the extreme flatness of the shell of the Lepton is of great advantage in enabling it not to get in the way of the Gebia as he scuttles up and down his burrow. Another species of Lepton is found on the coast of Florida in a precisely similar locality,[180] while a third species, occurring on the Oregon and California coasts, actually attaches itself to the inner surface of the abdomen of a Gebia.[181]
Fig. 32.—Ephippodonta Macdougalli Tate, S. Australia. A, Burrow of prawn, the X indicating the position of the mollusc; sp, sponge. B, Ventral view of Ephippodonta; by, byssus; f, foot; m, mantle; mm, fused mantle borders. C, View of interior of shells; h, hinge; m´m´, adductor muscles. (A × ½; B and C × 2.)
A very singular case of commensalism has been recently discovered with regard to a genus of Australian bivalve shells, Ephippodonta. This genus is never found except in the burrow of a species of prawn (Axius plectorhynchus Str.). For some reason at present unexplained, the burrow of this particular prawn appears to be exceedingly popular as a habitat for certain bivalves, for, besides two species of Ephippodonta, a Kellia and three Mylitta are found there, and there alone. Sometimes the prawn, when the rock is hard, builds a tunnel of mud upon it, at other times it excavates the soft calciferous sandstone. “This burrow is lined with a tenacious brown mud, composed of excrementitious matter; and, in addition to the mud lining, there is always more or less present an orange-coloured sponge which I have never found elsewhere. Upon the mud or sponge, and adhering very closely, are found the Ephippodonta. They quickly form a pit-like depression by means of their foot, and appear almost covered by the mud.” During the winter months[82] (March-July) the prawn appears to fill his burrow, possibly as a provision against stormy weather, with large quantities of minced seaweed, underneath which immense numbers of very young Ephippodonta are found living.[182] The extreme flatness of the Ephippodonta must be due to the same cause as the flatness of the Lepton noticed above, namely, the necessity of not impeding or interfering with the lively motions of the prawn. In the case of Lepton the two valves close completely and the shell is still very flat; in Ephippodonta, on the other hand, the same result is produced by the valves being opened to their widest possible extent. As in Entovalva, a continuation of the mantle covers the outer surface of the shell.
It is a familiar experience to the student, not only of the Mollusca, but of every branch of animal or vegetable life, to come across examples which exhibit certain slight deviations from the type form as usually understood. These deviations may be more or less pronounced, but, as a rule, a series of forms can be discovered, gradually leading up to or down from the type. The definition of what constitutes a species,—and, still more, the rigid application of such definition—will always remain a difficult task, so long as the personal element persists in him who defines.[183] What seems to one authority ample ground for distinction of species, another may regard as of comparatively trivial importance. The practical outcome of these divergent views is sufficiently illustrated by the attitude of Mr. F. P. Marrat on the one hand, and of what may be called the modern French school of conchologists on the other. Mr. Marrat holds, or held, that the great genus Nassa, of which more than 150 species are generally recognised, is one shell (species) in an endless variety of forms. The modern French school go to the other extreme, and apparently proceed upon the view that almost any difference in form, however slight, is sufficient to constitute a separate species.
It will be generally admitted, however, that some structural[83] difference in the organisation of the animal (as distinct from that of the shell alone) is necessary for the permanent constitution of specific rank.[184] What amount of structural difference is required, what particular organ or organs must exhibit this difference, will depend largely upon the idiosyncrasy of the observer. But if this, or something like this definition of a species be accepted, it will follow that a so-called ‘variety’ will be a form which exhibits differences from the type which do not amount to permanent structural differences in the organisation of the animal. The final court of appeal as to what affords sufficient evidence for ‘permanent structural differences’ will have to be, as with Aristotle of old, the judgment of the educated man.
It is, however, more to our present purpose to discuss the causes of variation than to lay down definitions of what variation is. One of the most obvious causes of variation lies in a change or changes in the environment. If we may assume, for the moment, that the type form of a species is the form which is the mean of all the extremes, and that this form is the resultant of all the varied forces brought to bear upon it, whether of food, climate, temperature, competition of numbers, soil, light, amount of water, etc., it will follow that any change in one or more of these forces, if continuous and considerable, any change, in other words, of the environment, will produce its effect upon the organism in question. And this effect will be for the better or for the worse, according to the particular nature of the change itself as tending towards, or away from, the optimum of environment for the species concerned. Hence may be produced varieties, more or less marked according to the gravity of the change, although it must be noted that at times a change apparently unimportant from our point of view, will produce very marked results upon the species. It is indeed scarcely possible to predict with any certainty, in the present state of our knowledge (beyond certain broad results) what will be the particular effect upon a species of any given change in its surroundings.
Effects of Change in the Environment as tending to produce Variation.
(a) Changes in Climate, Temperature, Elevation, etc.—In the eastern basin of the Baltic the marine Mollusca are much more[84] stunted than in the western.[185] For instance, Mytilus edulis near Kiel is 8–9 cm. long, while near Gothland it only attains a length of 3–4 cm. Mollusca living at only a shallow depth (e.g. Tellina balthica, Mya arenaria, Cardium edule) do not differ much in size in different parts of the Baltic, but in the far eastern basin the calcareous layers of the shells of Mya arenaria and Tellina balthica are extraordinarily thin, and disappear very rapidly after death, leaving only the cuticular membrane, still united by the ligament, in a perfect state of preservation. These remarkable variations are no doubt to a large extent due to the violent changes of temperature which are experienced in the Baltic, and by which the steady development of the animals in question is interrupted and thrown out of gear. The same species occur on the coasts of Greenland and Iceland, where they attain a considerably larger size than in the Baltic, in spite of the lower mean temperature, probably because their development is not interrupted by any sudden change from cold to heat or vice versâ.
Karl Semper has shown that Limnaea stagnalis is developed, lives and feeds best in a mean temperature of about 20° C. (= 68° F.). This mean, however, must not be the mean of two distant extremes, for the Limnaea cannot digest its food and grow in a temperature which is less than 14° or 15° C. (= 57° or 59° F.), or more than 30° to 32° C. (= 86° to 90° F.). In certain localities, therefore, the interruption to the growth of this species must be serious and prolonged, and may tend towards the production of more or less dwarfed varieties. Thus specimens from Malham Tarn, a lake in Yorkshire 1250 feet above the sea, are permanently dwarfed, and have a very thin and fragile shell. Limnaea peregra in the Pyrenees, Alps, and Himalayas is generally of a very delicate form and dwarfed habit, while the small variety known as lacustris occurs, according to Jeffreys, only in mountain lakes in Zetland, Scotland, Ireland, and N. England. Specimens brought by Mr. Bateson from lakes near the Sea of Aral, which are salt for some months and comparatively fresh for others, exhibit clearly the effect of changes in the environment (Figs. 33 and 34). Excess of heat produces similar results to excess of cold. L. peregra var. thermalis, found in the warm springs of the Pyrenees and the Vosges, and the var. geisericola,[85] from the hot water of the Iceland geysers, are alike thin and dwarfed forms.
Many instances may be given of ‘varieties due to locality.’ In some of these, the cause which predisposes towards variation can be inferred with some approach to certainty, in others we must be content to note the fact, without at present being able to perceive its explanation.
Fig. 33.—Four examples of Limnaea peregra Müll., from salt marshes near the Sea of Aral, showing different effects produced by abnormal conditions of life.
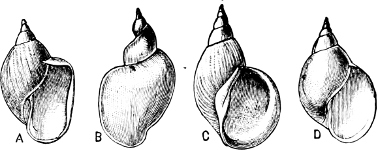
Fig. 34.—Four examples of Limnaea stagnalis L., from marshes in the Aral district which are salt for several months in the year, illustrating variation produced by changes in the environment. × ½.
Desert specimens of widely distributed species, e.g. Helix pomatia, H. niciensis, H. pisana, Leucochroa candidissima are much thicker than the type, and tend to lose all trace of coloured bands. These modifications are clearly the means of preventing evaporation of moisture, the dull white or grayish brown colour being calculated to absorb the smallest possible amount of heat. Desert shells in all parts of the world (e.g. N. Africa, Arabia, Central Asia, S. Africa, W. America) have been noticed to exhibit these peculiarities.
A very singular case of the reverse process, i.e. the production of darkened forms of shell through cold, has been noticed by Fischer as characteristic of the marine shells of the west coast of South America.[186] This melanism is especially noticeable in Trochus, Turbo, Chiton, Mitra, and Pleurotoma, and is attested by the specific names, not merely expressive of actual blackness (e.g. nigerrimus, ater, atramentarius, maurus), but also of a generally lugubrious tone (e.g. moestus, funebralis, tristis, lugubris, luctuosus). It is highly probable that this concurrence of specific melanism (which stands quite alone in the world) is[86] due to the cold polar current which impinges on the Chilian coasts, for the same genera occur on the opposite shores of the continent without exhibiting any trace whatever of this mournful characteristic.
It is a well-known fact, attested by many observers, that our common Limax agrestis as well as the young of Arion ater become decidedly darker in summer than in winter. If these slugs were accustomed to disport themselves in the sun, it might have been suggested that this increased darkness of colour tended to absorb more of the heat rays. But since this is not the case, the result is probably due to some unexplained effect of higher temperature. According to Lessona and Pollonera, the length of the keel in Limax arborum varies greatly in different parts of Italy, being shorter in specimens from low ground, but much longer in those inhabiting more elevated regions. The longer the keel, the more obscure the colouring becomes, so that in the Upper Alps of Piedmont individuals are practically black. Roebuck has observed that Scottish specimens of this same slug are much darker and less translucent than English forms. According to Simroth, our common black slug, Arion ater, is a northern type, which in more southern latitudes assumes the form known as A. rufus. Similarly Limax maximus “in its northern form cinereo-niger is almost wholly black, but in the more genial climate of Italy develops a series of brilliantly coloured and strikingly marked variations which have received numerous distinctive names from Italian limacologists.”[187] According to Scharff, however[188] (who regards the colours of slugs as in the main protective), these dark forms are by no means exclusively northern, being found equally on the parched plains of Spain and Portugal, and in the bleak climate of Norway. The same authority observes that similar forms occur both in the dry regions of E. Germany, and in the very humid district of western Ireland.
It appears unquestionable that marine genera from high northern latitudes are provided with shells of uniform colour, or whitish with a pale brown epidermis; spots, bands, or stripes seldom occur. The arctic forms of Buccinum, Trophon, Chrysodomus, Margarita, Crenella, Leda, Yoldia, Astarte illustrate this fact. In the more temperate seas of Europe, colours tend on the[87] whole to increase, although there are certain genera (e.g. Pecten) which are not more brightly coloured in Mediterranean than in Icelandic waters.
Land Mollusca inhabiting the mainland of a continent not unfrequently become smaller when they have spread to adjacent islands where perhaps the rainfall is less abundant or the soil and food-supply less nicely adjusted to their wants. Orthalicus undatus is decidedly larger on the mainland of S. America than on the adjacent islands of Trinidad and Grenada. Specimens of Bulimulus exilis from Barbados are invariably broader and more obese than those from S. Thomas, while those from the volcanic island of S. Lucia, where lime is deficient, are small and very slender. Streptaxis deformis, as occurring at Trinidad, is only half the size of specimens from Georgetown, Demerara.[189]
Certain localities appear, for some unexplained reason, to be particularly favourable to the production of albino varieties. The neighbourhood of Lewes, in Sussex, has produced no fewer than fourteen of these forms of land Mollusca and five of fresh-water.[190]
Our common Helix aspersa, as found near Bristol, is said to be ‘dark coloured’; about Western-super-mare ‘brown, with black markings’; near Bath ‘very pale and much mottled’; at Cheddar ‘very solid and large.’[191] Sometimes the same kind of variation is exhibited by different species in the same locality. Thus specimens of H. aspersa, H. nemoralis, and H. hortensis, taken from the same bank at Torquay, presented a straw-coloured tinge of ground colour, with red-brown bands or markings. Trochiform H. nemoralis and H. arbustorum, sinistral H. hortensis and H. aspersa, sinistral H. aspersa and H. virgata, and similarly banded forms of H. caperata and H. virgata, have been taken together.[192]
The immediate neighbourhood of the sea appears frequently to have the effect of dwarfing land Mollusca. Thus the var. conoidea of Helix aspersa, which is small, conical, with a compressed mouth, occurs ‘on sandhills and cliffs at the seaside.’ The varieties conica and nana of Helix hispida are found ‘near the sea.’ Helix virgata is exceedingly small in similar localities, and tends to become unicoloured. H. caperata var. Gigaxii, a[88] small depressed form, occurs at ‘Sandwich and Falmouth.’[193] Sometimes, however, the exact opposite is the case, for H. nemoralis var. major, which is ‘much larger’ than the type, occurs on ‘sandhills and downs’ and is ‘remarkably large in the I. of Arran, Co. Galway.’ The dwarf form of Limnaea peregra known as maritima appears to be confined to the neighbourhood of the sea.
Dwarfing of the shell seems frequently to be the result of an elevated locality, not perhaps so much as the direct consequence of purer air and less barometric pressure, as of changes in the character of the food supply and in the humidity of the air. Several species of Helix have a variety minor which is characteristic of an Alpine habitat. Helix arbustorum var. alpestris, which is scarcely two-thirds the size of the type, occurs on the Swiss Alps in the region of perpetual snow. Sometimes a very slight elevation is sufficient to produce the dwarfed form. At Tenby the type form of Helix pisana is scattered in countless numbers over the sandhills just above high-water mark. At the extreme western end of these sandhills rises abruptly to a height of over 100 feet the promontory known as Giltar Head, the vegetation of which is entirely distinct from that of the burrows below. There is a colony of H. pisana at the end of Giltar, all of which are devoid of the characteristic markings of the typical form, and most are dwarfed and stunted in growth.
Occasionally the same variety will be found to be produced by surroundings of very different nature. Thus the var. alpestris, of H. arbustorum mentioned above, besides being characteristic of high Alpine localities, also occurs abundantly in low marshes at Hoddesdon on the River Lea. Helix pulchella var. costata, according to Jeffreys, is found in dry and sandy places, often under loose stones and bricks on walls, while other authorities have noticed it in wet and dry localities quite indifferently.
Sometimes the production of a variety may be traced to the intrusion of some other organism. According to Brot, nine-tenths of the Limnaea peregra inhabiting a certain pond near Geneva, were, during one season, afflicted with a malformation of the base of the columella. This deformity coincided with the appearance, in the same waters, of extraordinary numbers of Hydra viridis. The next season, when the Hydra disappeared,[89] the next generation of Limnaea was found to have resumed its normal form.
It has been noticed that a form of Helix caperata with a flattened spire and wide umbilicus is restricted to tilled fields, especially the borders of clover fields, while a form with a more elevated spire and more compact whorls occurs exclusively in open downs and uncultivated places. The Rev. S. S. Pearce accounts[194] for this divergence by the explanation that the flatter spire enables the shell of the fields to creep about more easily under the leaves or matted weeds, seldom requiring to crawl up a stalk or stem, while on the short turf of the downs and pastures the smaller and more rounded shell enables the animal to manoeuvre in and out of the blades of grass, and even to crawl up them with considerable activity. The same writer endeavours to explain the causes which regulate the distribution of H. caperata var. ornata. He found that this variety (dark bands on a white ground) occurred almost exclusively on downs which were fed upon by sheep, associated with the common or mottled form, while the latter form alone occurred in localities where sheep were not accustomed to feed. Assuming then, as is probably the case, that sheep, in the course of their close pasturing, devour many small snails, he believes that individuals of the more conspicuous form ornata were more likely to be noticed, and therefore avoided, by the sheep, than the mottled form, which would more easily escape their observation. Hence the var. ornata is due to the advantage which strikingly coloured individuals obtained owing to their conspicuous habit, as compared with the typical form, which would be less readily detected.
(b) Changes in Soil, Station, Character of Water, etc.—A deficiency of lime in the composition of the soil of any particular locality produces very marked effects upon the shells of the Mollusca which inhabit it; they become small and very thin, occasionally almost transparent. The well-known var. tenuis of Helix aspersa occurs on downs in the Channel Islands where calcareous material is scarce. For similar reasons, H. arbustorum develops a var. fusca, which is depressed, very thin, and transparent, at Scilly, and also at Lunna I., E. Zetland.
Fig. 35.—19 specimens of Purpura lapillus L., Great Britain, illustrating variation.
(1) Felixstowe, sheltered coast; (2), (3) Newquay, on veined and coloured rock; (4) Herm, rather exposed; (5) Solent, very sheltered; (6) Land’s End, exposed rocks, small food supply; (7) Scilly, exposed rocks, fair food supply; (8) St. Leonards, flat mussel beds at extreme low water; (9) Robin Hood’s Bay, sheltered under boulders, good food supply; (10) Rhoscolyn, on oyster bed, 4–7 fath. (Macandrew); (11) Guernsey, rather exposed rocks; (12) Estuary of Conway, very sheltered, abundant food supply; (13), (14) Robin Hood’s Bay, very exposed rocks, poor food supply; (14) slightly monstrous; (15), (16), (17) Morthoe, rather exposed rocks, but abundant food supply; (18) St. Bride’s Bay; (19) L. Swilly, sheltered, but small food supply. All from the author’s collection, except (10).
The common dog-whelk (Purpura lapillus) of our own coasts[91] is an exceedingly variable species, and in many cases the variations may be shown to bear a direct relation to the manner of life (Fig. 35). Forms occurring in very exposed situations, e.g. Land’s End, outer rocks of the Scilly Is., coasts of N. Devon and Yorkshire, are stunted, with a short spire and relatively large mouth, the latter being developed in order to increase the power of adherence to the rock and consequently of resistance to wave force. On the other hand, shells occurring in sheltered situations, estuaries, narrow straits, or even on open coasts where there is plenty of shelter from the waves, are comparatively of great size, with a well-developed, sometimes produced spire, and a mouth small in proportion to the area of shell surface. In the accompanying figure, the specimens from the Conway estuary and the Solent (12, 5) well illustrate this latter form of shell, while that from exposed rocks is illustrated by the specimens from Robin Hood’s Bay (13, 14). Had these specimens occurred alone, or had they been brought from some distant and unexplored region, they must inevitably have been described as two distinct species.
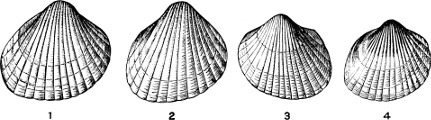
Fig. 36.—Valves of Cardium edule from the four upper terraces of Shumish Kul, a dry salt lake adjacent to the Aral Sea. (After Bateson.)
Mr. W. Bateson has made[195] some observations on the shells of Cardium edule taken from a series of terraces on the border of certain salt lakes which once formed a portion of the Sea of Aral. As these lakes gradually became dry, the water they contained became salter, and thus the successive layers of dead shells deposited on their borders form an interesting record of the progressive variation of this species under conditions which, in one respect at least, can be clearly appreciated. At the same time the diminishing volume of water, and the increasing average temperature, would not be without their effect. It was[92] found that the principal changes were as follows: the thickness, and consequently the weight, of the shells became diminished, the size of the beaks was reduced, the shell became highly coloured, and diminished considerably in size, and the breadth of the shells increased in proportion to their length (Fig. 36). Shells of the same species of Cardium, occurring in Lake Mareotis, were found to exhibit very similar variations as regards colour, size, shape, and thickness.
Unio pictorum var. compressa occurs near Norwich at two similar localities six or seven miles distant from one another, under circumstances which tend to show that similar conditions have produced similar results. The form occurs where the river, by bending sharply in horse-shoe shape, causes the current to rush across to the opposite side and form an eddy near the bank on the outside of the bend. Just at the edge of the sharp current next the eddy the shells are found, the peculiar form being probably due to the current continually washing away the soft particles of mud and compelling the shell to elongate itself in order to keep partly buried at the bottom.[196]
The rivers Ouse and Foss, which unite just below York, are rivers of strikingly different character, the Ouse being deep, rapid, with a bare, stony bottom, and little vegetable growth, and receiving a good deal of drainage, while the Foss is shallow, slow, muddy, full of weeds and with very little drainage. In the Foss, fine specimens of Anodonta anatina occur, lustrous, with beautifully rayed shells. A few yards off, in the Ouse, the same species of Anodonta is dull brown in colour, its interior clouded, the beaks and epidermis often deeply eroded. Precisely the same contrast is shown in specimens of Unio tumidus, taken from the same rivers, Ouse specimens being also slightly curved in form. Just above Yearsley Lock in the Foss, Unio tumidus occurs, but always dwarfed and malformed, a result probably due to the effect of rapidly running water upon a species accustomed to live in still water.[197] Simroth records the occurrence of remarkably distorted varieties in two species of Aetheria which lived in swift falls of the River Congo.[198]
A variety of Limnaea peregra with a short spire and rather strong, stoutly built shell occurs in Lakes Windermere, Derwentwater,[93] and Llyn-y-van-fach. It lives adhering to stones in places where there are very few weeds, its shape enabling it to withstand the surf of these large lakes, to which the ordinary form would probably succumb.[199]
Scalariform specimens of Planorbis are said to occur most commonly in waters which are choked by vegetation, and it has been shown that this form of shell is able to make its way through masses of dense weed much more readily than specimens of normal shape.
Continental authorities have long considered Limnaea peregra and L. ovata as two distinct species. Hazay, however, has succeeded in rearing specimens of so-called peregra from the ova of ovata, and so-called ovata from the ova of peregra, simply by placing one species in running water, and the other in still water.
According to Mr. J. S. Gibbons[200] certain species of Littorina, in tropical and sub-tropical regions, are confined to water more or less brackish, being incapable of living in pure salt water. “I have met,” says Mr. Gibbons, “with three of these species, and in each case they have been distinguished from the truly marine species by the extreme (comparative) thinness of their shells, and by their colouring being richer and more varied; they are also usually more elaborately marked. They are to be met with under three different conditions—(1) in harbours and bays where the water is salt with but a slight admixture of fresh water; (2) in mangrove swamps where salt and fresh water mix in pretty equal volume; (3) on dry land, but near a marsh or the dry bed of one.
“L. intermedia Reeve, a widely diffused E. African shell, attaches itself by a thin pellicle of dried mucus to grass growing by the margin of slightly brackish marshes near the coast, resembling in its mode of suspension the Old World Cyclostoma. I have found it in vast numbers in situations where, during the greater part of the year, it is exposed to the full glare of an almost vertical sun, its only source of moisture being a slight dew at night-time. The W. Indian L. angulifera Lam., and a beautifully coloured E. African species (? L. carinifera), are found in mangrove swamps; they are, however, less independent of salt water than the last.”
[94]
Mr. Gibbons goes on to note that brackish water species (although not so solid as truly marine species) tend to become more solid as the water they inhabit becomes less salt. This is a curious fact, and the reverse of what one would expect. Specimens of L. intermedia on stakes at the mouth of the Lorenço Marques River, Delagoa Bay, are much smaller, darker, and more fragile, than those living on grass a few hundred yards away. L. angulifera is unusually solid and heavy at Puerto Plata (S. Domingo) among mangroves, where the water is in a great measure fresh; at Havana and at Colon, where it lives on stakes in water but slightly brackish, it is thinner and smaller and also darker coloured.
(c) Changes in the Volume of Water.—It has long been known that the largest specimens, e.g. of Limnaea stagnalis and Anodonta anatina, only occurred in pieces of water of considerable size. Recent observation, however, has shown conclusively that the volume of water in which certain species live has a very close relation to the actual size of their shells, besides producing other effects. Lymnaea megasoma, when kept in an aquarium of limited size, deposited eggs which hatched out; this process was continued in the same aquarium for four generations in all, the form of the shell of the last generation having become such that an experienced conchologist gave it as his opinion that the first and last terms of the series could have no possible specific relation to one another. The size of the shell became greatly diminished, and in particular the spire became very slender.[201]
The same species being again kept in an aquarium under similar conditions, it was found that the third generation had a shell only four-sevenths the length of their great grandparents. It was noticed also that the sexual capacities of the animals changed as well. The liver was greatly reduced, and the male organs were entirely lost.[202]
K. Semper conducted some well-known experiments bearing on this point. He separated[203] specimens of Limnaea stagnalis from the same mass of eggs as soon as they were hatched, and placed them simultaneously in bodies of water varying in volume from 100 to 2000 cubic centimetres. All the other conditions of life, and especially the food supply, were kept at the[95] known optimum. He found, in the result, that the size of the shell varied directly in proportion to the volume of the water in which it lived, and that this was the case, whether an individual specimen was kept alone in a given quantity of water, or shared it with several others. At the close of 65 days the specimens raised in 100 cubic cm. of water were only 6 mm. long, those in 250 cubic cm. were 9 mm. long, those in 600 cubic cm. were 12 mm. long, while those kept in 2000 cubic cm. attained a length of 18 mm. (Fig. 37).
An interesting effect of a sudden fall of temperature was noticed by Semper in connection with the above experiments. Vessels of unequal size, containing specimens of the Limnaea, happened to stand before a window at a time when the temperature suddenly fell to about 55° F. The sun, which shone through the window, warmed the water in the smaller vessels, but had no effect upon the temperature of the larger. The result was, that the Limnaea in 2000 cubic cm., which ought to have been 10 mm. long when 25 days old, were scarcely longer, at the end of that period, than those which had lived in the smaller vessels, but whose water had been sufficiently warm.
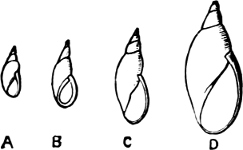
Fig. 37.—Four equally old shells of Limnaea stagnalis, hatched from the same mass of ova, but reared in different volumes of water: A in 100, B in 250, C in 600, and D in 2000 cubic centimetres. (After K. Semper.)
[96]
The employment of shells as a medium of exchange was exceedingly common amongst uncivilised tribes in all parts of the world, and has by no means yet become obsolete. One of the commonest species thus employed is the ‘money cowry’ (Cypraea moneta, L.), which stands almost alone in being used entire, while nearly all the other forms of shell money are made out of portions of shells, thus requiring a certain amount of labour in the process of formation.
One of the earliest mentions of the cowry as money occurs in an ancient Hindoo treatise on mathematics, written in the seventh century A.D. A question is propounded thus: ‘the ¼ of 1/16 of ⅕ of ¾ of ⅔ of ½ a dramma was given to a beggar by one from whom he asked an alms; tell me how many cowry shells the miser gave.’ In British India about 4000 are said to have passed for a shilling, but the value appears to differ according to their condition, poor specimens being comparatively worthless. According to Reeve[204] a gentleman residing at Cuttack is said to have paid for the erection of his bungalow entirely in cowries. The building cost him 4000 Rs. sicca (about £400), and as 64 cowries = 1 pice, and 64 pice = 1 rupee sicca, he paid over 16,000,000 cowries in all.
Cowries are imported to England from India and other places for the purposes of exportation to West Africa, to be exchanged for native products. The trade, however, appears to be greatly on the decrease. At the port of Lagos, in 1870, 50,000 cwts. of cowries were imported.[205]
[97]
A banded form of Nerita polita was used as money in certain parts of the South Pacific. The sandal-wood imported into the China market is largely obtained from the New Hebrides, being purchased of the natives in exchange for Ovulum angulosum, which they especially esteem as an ornament. Sometimes, as in the Duke of York group, the use of shell money is specially restricted to certain kinds of purchase, being employed there only in the buying of swine.
Among the tribes of the North-West coasts of America the common Dentalium indianorum used to form the standard of value, until it was superseded, under the auspices of the Hudson’s Bay Company, by blankets. A slave was valued at a fathom of from 25 to 40 of these shells, strung lengthwise. Inferior or broken specimens were strung together in a similar way, but were less highly esteemed; they corresponded more to our silver and copper coins, while the strings of the best shells represented gold.
The wampum of the eastern coast of North America differed from all these forms of shell money, in that it required a laborious process for its manufacture. Wampum consisted of strings of cylindrical beads, each about a quarter of an inch in length and half that breadth. The beads were of two colours, white and purple, the latter being the more valuable. Both were formed from the common clam, Venus mercenaria, the valves of which are often stained with purple at the lower margins, while the rest of the shell is white. Cut small, ground down, and pierced, these shells were converted into money, which appears to have been current along the whole sea-board of North America from Maine to Florida, and on the Gulf Coast as far as Central America, as well as among the inland tribes east of the Mississippi. Another kind of wampum was made from the shells of Busycon carica and B. perversum. By staining the wampum with various colours, and disposing these colours in belts in various forms of arrangement, the Indians were able to preserve records, send messages, and keep account of any kind of event, treaty, or transaction.
Another common form of money in California was Olivella biplicata, strung together by rubbing down the apex. Button-shaped disks cut from Saxidomus arata and Pachydesma crassatelloides, as well as oblong pieces of Haliotis, were employed for[98] the same purpose, when strung together in lengths of several yards.
“There is a curious old custom,” writes Mr. W. Anderson Smith,[206] “that used formerly to be in use in this locality [the western coast of Scotland], and no doubt was generally employed along the sea-board, as the most simple and ready means of arrangement of bargains by a non-writing population. That was, when a bargain was made, each party to the transaction got one half of a bivalve shell—such as mussel, cockle, or oyster—and when the bargain was implemented, the half that fitted exactly was delivered up as a receipt! Thus a man who had a box full of unfitted shells might be either a creditor or a debtor; but the box filled with fitted shells represented receipted accounts. Those who know the difficulty of fitting the valves of some classes of bivalves will readily acknowledge the value of this arrangement.”
Shells are employed for use and for ornament by savage—and even by civilised—tribes in all parts of the world. The natives of Fiji thread the large Turbo argyrostoma and crenulatus as weights at the edge of their nets, and also employ them as sinkers. A Cypraea tigris cut into two halves and placed round a stone, with two or three showy Oliva at the sides, is used as a bait for cuttles. Avicula margaritifera is cut into scrapers and knives by this and several other tribes. Breast ornaments of Chama, grouped with Solarium perspectivum and Terebra duplicata are common among the Fijians, who also mount the Avicula on a backing of whales’ teeth sawn in two, for the same purpose. The great Orange Cowry (Cypraea aurantiaca) is used as a badge of high rank among the chieftains. One of the most remarkable Fijian industries is the working of whales’ teeth to represent this cowry, as well as the commoner C. talpa, which is more easily imitated.
Among the Solomon islanders, cowries are used to ornament their shields on great field days, and split cowries are worn as a necklace, to represent human teeth. Small bunches of Terebellum subulatum are worn as earrings, and a large valve of Avicula is employed as a head ornament in the centre of a fillet. The same islanders ornament the raised prows of their canoes, as well as the inside of the stern-post, with a long row of single Natica.
[99]
The native Papuans employ shells for an immense variety of purposes. Circlets for the head are formed of rows of Nassa gibbosula, rubbed down till little but the mouth remains. Necklaces are worn which consist of strings of Oliva, young Avicula, Natica melanostoma, opercula of Turbo, and valves of a rich brown species of Cardium, pendent at the end of strings of the seeds known as Job’s tears. Struthiolaria is rubbed down until nothing but the mouth is left, and worn in strings round the neck. This is remarkable, since Struthiolaria is not a native Papuan shell, and indeed occurs no nearer than New Zealand. Sections of Melo are also worn as a breast ornament, dependent from a necklace of cornelian stones. Cypraea erosa is used to ornament drinking bowls, and Ovulum ovum is attached to the native drums, at the base of a bunch of cassowary feathers, as well as being fastened to the handle of a sago-beater.
In the same island, the great Turbo and Conus millepunctatus are ground down to form bracelets, which are worn on the biceps. The crimson lip of Strombus luhuanus is cut into beads and perforated for necklaces. Village elders are distinguished by a single Ovulum verrucosum, worn in the centre of the forehead. The thick lip of Cassis cornuta is ground down to form nose pieces, 4½ inches long. Fragments of a shell called Kaïma (probably valves of a large Spondylus) are worn suspended from the ears, with little wisps of hair twisted up and thrust through a hole in the centre. For trumpets, Cassis cornuta, Triton tritonis, and Ranella lampas are used, with a hole drilled as a mouthpiece in one of the upper whorls. Valves of Batissa, Unio, and Mytilus are used as knives for peeling yams. Spoons for scooping the white from the cocoa-nut are made from Avicula margaritifera. Melo diadema is used as a baler in the canoes.[207]
In the Sandwich Islands Melampus luteus is worn as a necklace, as well as in the Navigator Islands. A very striking necklace, in the latter group, is formed of the apices of a Nautilus, rubbed down to show the nacre. The New Zealanders use the green opercula of a Turbo, a small species of Venus, and Cypraea asellus to form the eyes of their idols. Fish-hooks are made throughout the Pacific of the shells of Avicula and[100] Haliotis, and are sometimes strengthened by a backing made of the columella of Cypraea arabica. Small axe-heads are made from Terebra crenulata ground down (Woodlark I.), and larger forms are fashioned from the giant Tridacna (Fiji).
Shells are used to ornament the elaborate cloaks worn by the women of rank in the Indian tribes of South America. Specimens of Ampullaria, Orthalicus, Labyrinthus, and Bulimulus depend from the bottom and back of these garments, while great Bulimi, 6 inches long, are worn as a breast ornament, and at the end of a string of beads and teeth.[208]
The chank-shell (Turbinella rapa) is of especial interest from its connexion with the religion of the Hindoos. The god Vishnu is represented as holding this shell in his hand, and the sinistral form of it, which is excessively rare, is regarded with extraordinary veneration. The chank appears as a symbol on the coins of some of the ancient Indian Empires, and is still retained on the coinage of the Rajah of Travancore.
The chief fishery of the chank-shell is at Tuticorin, on the Gulf of Manaar, and is conducted during the N. E. monsoon, October-May. In 1885–86 as many as 332,000 specimens were obtained, the net amount realised being nearly Rs.24,000. In former days the trade was much more lucrative, 4 or 5 millions of specimens being frequently shipped. The government of Ceylon used to receive £4000 a year for licenses to fish, but now the trade is free. The shells are brought up by divers from 2 or 3 fathoms of water. In 1887 a sinistral specimen was found at Jaffna, which sold for Rs.700.[209] Nearly all the shells are sent to Dacca, where they are sliced into bangles and anklets to be worn by the Hindoo women.
Perhaps the most important industry which deals only with the shells of Mollusca is that connected with the ‘pearl-oyster.’ The history of the trade forms a small literature in itself. It must be sufficient here to note that the species in question is not an ‘oyster,’ properly so called, but an Avicula (margaritifera Lam.). The ‘mother-of-pearl,’ which is extensively employed for the manufacture of buttons, studs, knife-handles, fans, card-cases, brooches, boxes, and every kind of inlaid work, is the[101] internal nacreous laminae of the shell of this species. The most important fisheries are those of the Am Islands, the Soo-loo Archipelago, the Persian Gulf, the Red Sea, Queensland, and the Pearl Islands in the Bay of Panama. The shell also occurs in several of the groups of the South Pacific—the Paumotu, Gambier and Navigator Islands, Tahiti being the centre of the trade—and also on the coasts of Lower California.[210]
Pearls are the result of a disease in the animal of this species of Avicula and probably in all other species within which they occur. When the Avicula is large, well formed, and with ample space for individual development, pearls scarcely occur at all, but when the shells are crowded together, and become humped and distorted, as well as affording cover for all kinds of marine worms and parasitic creatures, then pearls are sure to be found. Pearls of inferior value and size are also produced by Placuna placenta, many species of Pinna, the great Tridacna, the common Ostrea edulis, and several other marine bivalves. They are not uncommon in Unio and Anodonta, and the common Margaritana margaritifera of our rapid streams is still said to be collected, in some parts of Wales, for the purpose of extracting its small ‘seed-pearls.’ Pink pearls are obtained from the giant conch-shell of the West Indies (Strombus gigas), as well as from certain Turbinella.
In Canton, many houses are illuminated almost entirely by skylights and windows made of shells, probably the semitransparent valves of Placuna placenta. In China lime is commonly made of ground cockle-shells, and, when mixed with oil, forms an excellent putty, used for cementing coffins, and in forming a surface for the frescoes with which the gables of temples and private houses are adorned. Those who suffer from cutaneous diseases, and convalescents from small-pox, are washed in Canton with the water in which cockles have been boiled.[211]
A recent issue of the Peking Gazette contains a report from the outgoing Viceroy of Fukhien, stating that he had handed over the insignia of office to his successor, including inter alia the conch-shell bestowed by the Throne. A conch-shell with a whorl turning to the right, i.e. a sinistral specimen, is supposed when blown to have the effect of stilling the waves, and hence is[102] bestowed by the Emperor upon high officers whose duties oblige them to take voyages by sea. The Viceroy of Fukhien probably possesses one of these shells in virtue of his jurisdiction over Formosa, to which island periodical visits are supposed to be made.[212]
Shells appear to be used occasionally by other species besides man. Oyster-catchers at breeding time prepare a number of imitation nests in the gravel on the spit of land where they build, putting bits of white shell in them to represent eggs.[213] This looks like a trick in order to conceal the position of the true nest. According to Nordenskjöld, when the eider duck of Spitzbergen has only one or two eggs in its nest, it places a shell of Buccinum glaciale beside them. The appropriation of old shells by hermit-crabs is a familiar sight all over the world. Perhaps it is most striking in the tropics, where it is really startling, at first experience, to meet—as I have done—a large Cassis or Turbo, walking about in a wood or on a hill side at considerable distances from the sea. A Gephyrean (Phascolion strombi) habitually establishes itself in the discarded shells of marine Mollusca. Certain Hymenoptera make use of dead shells of Helix hortensis in which they build their cells.[214] Magnus believes that in times when heavy rains prevail, and the usual insects do not venture out, certain flowers are fertilised by snails and slugs crawling over them, e.g. Leucanthemum vulgare by Limax laevis.[215]
Mollusca as Food for Man.—Probably there are few countries in the world in which less use is made of the Mollusca as a form of food than in our own. There are scarcely ten native species which can be said to be at all commonly employed for this purpose. Neighbouring countries show us an example in this respect. The French, Italians, and Spanish eat Natica, Turbo, Triton, and Murex, and, among bivalves, Donax, Venus, Lithodomus, Pholas, Tapes, and Cardita, as well as the smaller Cephalopoda. Under the general designation of clam the Americans eat Venus mercenaria, Mya arenaria, and Mactra solidissima. In the Suez markets are exposed for sale Strombus and Melongena, Avicula and Cytherea. At Panama Donax and Solen are[103] delicacies, while the natives also eat the great Murex and Pyrula, and even the huge Arca grandis, which lives embedded in the liquid river mud.
The common littoral bivalves seem to be eaten in nearly all countries except our own, and it is therefore needless to enumerate them. The Gasteropoda, whose habits are scarcely so cleanly, seem to require a bolder spirit and less delicate palate to venture on their consumption.
The Malays of the East Indian islands eat Telescopium fuscum and Pyrazus palustris, which abound in the mangrove swamps. They throw them on their wood fires, and when they are sufficiently cooked, break off the top of the spire and suck the animal out through the opening. Haliotis they take out of the shell, string together, and dry in the sun. The lower classes in the Philippines eat Arca inaequivalvis, boiling them as we do mussels.[216] In the Corean islands a species of Monodonta and another of Mytilus are quite peppery, and bite the tongue; our own Helix revelata, as I can vouch from personal experience, has a similar flavour. Fusus colosseus, Rapana bezoar, and Purpura luteostoma are eaten on the southern coasts of China; Strombus luhuanus, Turbo chrysostomus, Trochus niloticus, and Patella testudinaria, by the natives of New Caledonia; Strombus gigas and Livona pica in the West Indies; Turbo niger and Concholepas peruvianus on the Chilian coasts; four species of Strombus and Nerita, one each of Purpura and Turbo, besides two Tridacna and one Hippopus, by the natives of British New Guinea. West Indian negroes eat the large Chitons which are abundant on their rocky coasts, cutting off and swallowing raw the fleshy foot, which they call ‘beef,’ and rejecting the viscera. Dried cephalopods are a favourite Chinese dish, and are regularly exported to San Francisco, where the Chinamen make them into soup. The ‘Challenger’ obtained two species of Sepia and two of Loligo from the market at Yokohama.
The insipidity of fresh-water Mollusca renders them much less desirable as a form of food. Some species of Unionidae, however, are said to be eaten in France. Anodonta edulis is specially cultivated for food in certain districts of China, and the African Aetheriae are eaten by negroes. Navicella and Neritina are eaten in Mauritius, Ampullaria and Neritina in Guadeloupe, and Paludina in Cambodia.
[104]
The vast heaps of empty shells known as ‘kitchen-middens,’ occur in almost every part of the world. They are found in Scotland, Denmark, the east and west coasts of North America, Brazil, Tierra del Fuego, Australia and New Zealand, and are sometimes several hundred yards in length. They are invariably composed of the edible shells of the adjacent coast, mixed with bones of Mammals, birds, and fish. From their great size, it is believed that many of them must have taken centuries to form.
Pre-eminent among existing shell-fish industries stands the cultivation of the oyster and the mussel, a more detailed account of which may prove interesting.
The cultivation of the oyster[217] as a luxury of food dates at least from the gastronomic age of Rome. Every one has heard of the epicure whose taste was so educated that
The first artificial oyster-cultivator on a large scale appears to have been a certain Roman named Sergius Orata, who lived about a century B.C. His object, according to Pliny the elder,[219] was not to please his own appetite so much as to make money by ministering to the appetites of others. His vivaria were situated on the Lucrine Lake, near Baiae, and the Lucrine oysters obtained under his cultivation a notoriety which they never entirely lost, although British oysters eventually came to be more highly esteemed. He must have been a great enthusiast in his trade, for on one occasion when he became involved in a law-suit with one of the riparian proprietors, his counsel declared that Orata’s opponent made a great mistake if he expected to damp his ardour by expelling him from the lake, for, sooner than not grow oysters at all, he would grow them upon the roof of his house.[220] Orata’s successors in the business seem to have understood the secret of planting young oysters in new beds, for[105] we are told that specimens brought from Brundisium and even from Britain were placed for a while in the Lucrine Lake, to fatten after their long journey, and also to acquire the esteemed “Lucrine flavour.”
Oysters are ‘in season’ whenever there is an ‘r’ in the month, in other words, from September to April. ‘Mensibus erratis,’ as the poet has it, ‘vos ostrea manducatis!’ It has been computed that the quantity annually produced in Great Britain amounts to no less than sixteen hundred million, while in America the number is estimated at five thousand five hundred million, the value being over thirteen million dollars, and the number of persons employed fifty thousand. Arcachon, one of the principal French oyster-parks, has nearly 10,000 acres of oyster beds, the annual value being from eight to ten million francs; in 1884–85, 178,359,000 oysters were exported from this place alone. In the season 1889–90, 50,000 tons of oysters were consumed in London.
Few will now be found to echo the poet Gay’s opinion:
There were halcyon days in England once, when oysters were to be procured at 8d. the bushel. Now it costs exactly that amount before a bushel, brought up the Thames, can even be exposed for sale at Billingsgate (4d. porterage, 4d. market toll), and prime Whitstable natives average from 3½d. to 4d. each. The principal causes of this rise in prices, apart from the increased demand, are (1) over-dredging; (2) ignorant cultivation, and to these may be added (3) the effect of bad seasons in destroying young oysters, or preventing the spat from maturing. Our own principal beds are those at Whitstable, Rochester, Colchester, Milton (famous for its ‘melting’ natives), Faversham, Queenborough, Burnham, Poole, and Carlingford in Co. Down, and Newhaven, near Edinburgh.
The oyster-farms at Whitstable, public and private, extend over an area of more than 27 square miles. The principal of these is a kind of joint-stock company, with no other privilege of entrance except birth as a free dredgeman of the town. When a holder dies, his interest dies with him. Twelve directors,[106] known as “the Jury,” manage the affairs of the company, which finds employment for several thousand people, and sometimes turns over as much as £200,000 a year. The term ‘Natives,’ as applied to these Whitstable or to other English oysters, requires a word of explanation. A ‘Native’ oyster is simply an oyster which has been bred on or near the Thames estuary, but very probably it may be developed from a brood which came from Scotland or some other place at a distance. For some unexplained reason, oysters bred on the London clay acquire a greater delicacy of flavour than elsewhere. The company pay large sums for brood to stock their own grounds, since there can be no certainty that the spat from their own oysters will fall favourably, or even within their own domains at all. Besides purchases from other beds, the parks are largely stocked with small oysters picked up along the coast or dredged from grounds public to all, sometimes as much as 50s. a bushel being paid for the best brood. It is probably this system of transplanting, combined with systematic working of the beds, which has made the Whitstable oyster so excellent both as to quality and quantity of flesh. The whole surface of the ‘layings’ is explored every year by the dredge, successive portions of the ground being gone over in regular rotation, and every provision being made for the well-being of the crop, and the destruction of their enemies. For three days of every week the men dredge for ‘planting,’ i.e. for the transference of suitable specimens from one place to another, the separation of adhering shells, the removal of odd valves and of every kind of refuse, and the killing off of dangerous foes. On the other three days they dredge for the market, taking care only to lift such a number as will match the demand.
The Colne beds are natural beds, as opposed to the majority of the great working beds, which are artificial. They are the property of the town of Colchester, which appoints a water-bailiff to manage the concern. Under his direction is a jury of twelve, who regulate the times of dredging, the price at which sales are to be made, and are generally responsible for the practical working of the trade. Here, and at Faversham, Queenborough, Rochester, and other places, ‘natives’ are grown which rival those of Whitstable.
There can be no question, however, that the cultivation of oysters by the French is far more complete and efficient than[107] our own, and has reached a higher degree of scientific perfection combined with economy and solid profits. And yet, between 40 and 50 years ago, the French beds were utterly exhausted and unproductive, and showed every sign of failure and decay. It was in 1858 that the celebrated beds on the Ile de Ré, near Rochelle, were first started. Their originator was a certain shrewd stone-mason, by name Boeuf. He determined to try, entirely on his own account, whether oysters could not be made to grow on the long muddy fore-shore which is left by the ebb of the tide. Accordingly, he constructed with his own hands a small basin enclosed by a low wall, and placed at the bottom a number of stones picked out of the surrounding mud, stocking his ‘parc’ with a few bushels of healthy young brood. The experiment was entirely successful, in spite of the jeers of his neighbours, and Boeuf’s profits, which soon began to mount up at an astonishing rate, induced others to start similar or more extensive farms for themselves. The movement spread rapidly, and in a few years a stretch of miles of unproductive mud banks was converted into the seat of a most prosperous industry. The general interests of the trade appear to be regulated in a similar manner to that at Whitstable; delegates are appointed by the various communities to watch over the business as a whole, while questions affecting the well-being of oyster-culture are discussed in a sort of representative assembly.
At the same time as Boeuf was planting his first oysters on the shores of the Ile de Ré, M. Coste had been reporting to the French government in favour of such a system of ostreiculture as was then practised by the Italians in the old classic Lakes Avernus and Lucrinus. The principle there adopted was to prevent, as far as possible, the escape of the spat from the ground at the time when it is first emitted by the breeding oyster. Stakes and fascines of wood were placed in such a position as to catch the spat and give it a chance of obtaining a hold before it perished or was carried away into the open sea. The old oyster beds in the Bay of St. Brieuc were renewed on this principle, banks being constructed and overlaid with bundles of wood to prevent the escape of the new spat. The attempt was entirely successful, and led to the establishment or re-establishment of those numerous parcs, with which the French coast is studded from Brest to the Gironde. The principal[108] centres of the industry are Arcachon, Auray, Cancale, and la Teste.
It is at Marennes, in Normandy, that the production of the celebrated ‘green oyster’ is carried out, that especial luxury of the French epicure. Green oysters are a peculiarly French taste, and, though they sometimes occur on the Essex marshes, there is no market for them in England. The preference for them, on the continent, may be traced back as early as 1713, when we find a record of their having been served up at a supper given by an ambassador at the Hague. Green oysters are not always green, it is only after they are placed in the ‘claires,’ or fattening ponds, that they acquire the hue; they never occur in the open sea. The green colour does not extend over the whole animal, but is found only in the branchiae and labial tentacles, which are of a deep blue-green. Various theories have been started to explain the ‘greening’ of the mollusc; the presence of copper in the tanks, the chlorophyll of marine algae, an overgrowth of some parasite, a disease akin to liver complaint, have all found their advocates. Prof. Lankester seems to have established[221] the fact,—which indeed had been observed 70 years before by a M. Gaillon,—that the greening is due to the growth of a certain diatom (Navicula ostrearia) in the water of the tanks. This diatom, which is of a deep blue-green colour, appears from April to June, and in September. The oyster swallows quantities of the Navicula; the pigment enters the blood in a condition of chemical modification, which makes it colourless in all the other parts of the body, but when the blood reaches the gills the action of the secretion cells causes the blue tint to be restored. The fact that the colour is rather green than blue in the gills, which are yellowish brown, is due to certain optical conditions.
Not till the young white oyster has been steeped for several years in the muddy waters of the ‘claires’ does it acquire the proper tint to qualify it for the Parisian restaurant. The ‘claires’ are each about 100 feet square, surrounded by low broad banks of earth, about 3 feet high and 6 feet thick at the base. Before the oysters are laid down, the gates which admit the tide are carefully opened and shut a great many times, in order to collect a sufficient amount of the Navicula. When this is done, the beds are formed, and are not again overflowed by[109] the sea, except at very high tides. The oysters are shifted from one ‘claire’ to another, in order to perfect the ‘greening’ process. About fifty million of these ‘huitres de Marennes’ are produced annually, yielding a revenue of 2,500,000 francs.
It appears, from the experience of one of the most enthusiastic of French oyster-growers (Dr. Kemmerer), that oysters grow best in muddy water, and breed best in clear water. Thus the open sea is the place where the spat should fall and be secured, and, as soon as it is of a suitable size, it should be transferred to the closed tank or reservoir, where it will find the quiet and the food (confervae, infusoria, minute algae) which are so requisite for its proper growth. In muddy ground the animal and phosphorous matter increases, and the flesh becomes fatter and more oily. A sudden change from the clear sea-water to the muddy tank is inadvisable, and thus a series of shiftings through tanks with water of graduated degrees of nourishment is the secret of proper oyster cultivation.
The American oyster trade is larger even than the French. The Baltimore oyster beds in the Chesapeake River and its tributaries cover 3000 acres, and produce an annual crop of 25 million bushels, as many as 100,000 bushels being sometimes taken from Chesapeake Bay in a single day. Baltimore is the centre of the tinned oyster trade, while that in raw oysters centres in New York. Most of the beds whose produce is carried to New York are situated in New Jersey, Connecticut, Delaware, or Virginia. The laws of these states do not allow the beds to be owned by any but resident owners, and the New York dealers have consequently to form fictitious partnerships with residents near the various oyster beds, supply them with money to buy the beds and plant the oysters, and then give them a share in the profits. It has been estimated that from the Virginia beds 4,000,000 bushels of oysters are carried every year to Fair Haven in New England, 4,000,000 to New York, 3,000,000 to Providence, and 2,000,000 each to Boston, Philadelphia, and Baltimore. The American ‘native’ (O. virginica) is a distinct species from our own, being much larger and longer in proportion to its breadth; it is said to be also much more prolific.
According to Milne-Edwards,[222] in the great oyster parks on[110] the coasts of Calvados, the oysters are educated to keep their shells closed when out of water, and so retain water enough inside to keep their gills moist, and arrive at their destination in good condition. As soon as an oyster is taken out of the sea, it closes its shells, and keeps them closed until the shock of removal has passed away, or perhaps until the desirability of a fresh supply of water suggests itself. The men take advantage of this to exercise the oysters, removing them from the sea for longer and longer periods. In time this has the desired effect; the well-educated mollusc learns that it is hopeless to ‘open’ when out of the water, and so keeps his shell closed and his gills moist, and his general economy in good condition.
Oysters have been known to live entirely out of water for a considerable time. Prof. Verrill once noticed[223] a large cluster of oysters attached to an old boot, hanging outside a fish-shop in Washington. They had been taken out of the water on about 10th December, and on 25th February following some of the largest were still alive. It was noticed that all those which survived had the hinge upward and the ventral edge downward, this being the most favourable position possible for the retention of water within the gill-cavity, since the edge of the mantle would pack against the margins of the shell, and prevent the water from leaking away.
Such a succulent creature as the oyster has naturally many enemies. One of the worst of these is the ravenous Starfish, or Five-finger. His omnivorous capacities are well described by a clever writer and shrewd observer of nature: “Here is one doubled up like a sea-urchin, brilliant of hue, and when spread out quite 16 inches in diameter; where, and oh where, can you obtain a prey? The hoe we carry is thrust out and the mass dragged shorewards, when the rascal disgorges two large dog-whelks he has been in the process of devouring. We feel a comfortable glow of satisfaction to think that this enemy of our oyster-beds is also the enemy of our other enemy, this carnivorous borer. Here, quite close alongside, is another, only inferior in size, and we drag him ashore likewise, to find that the fellow has actually had the courage and audacity to suck the contents out of a large horse-mussel (Modiola), the strong muscle alone remaining undevoured. We proceed along but a short way[111] when we meet with still another in the curled-up condition in which they gorge themselves, and as we drag it shorewards the shell of a Tapes pullastra drops from the relaxing grasp of the ogre. Slowly the extended stomach returns to its place, and the monster settles back to an uncomfortable after-dinner siesta on an exposed boulder; for the starfish wraps its turned-out stomach around the prey it has secured, in place of attempting to devour the limey covering in which most of its game is protected. Once the mouth of the shell is enclosed in the stomach of the starfish, the creature soon sickens, the hinge-spring relaxes its hold, and the shell opening permits the starfish to suck out the gelatinous contents, and cast free the calcareous skeleton.”[224]
According to other observers the starfish seizes the oyster with two of his fingers, while with the other three he files away the edge of the flat or upper valve until the points of contact with the round valve are reduced almost to nothing; then he can introduce an arm, and the rest is easy work. Others suggest that the starfish suffocates the oyster by applying two of its fingers so closely to the edge of the valves that the oyster is unable to open them; after a while the vital powers relax and the shell gapes. The Rev. J. G. Wood holds[225] that the starfish pours a secretion from its mouth which “paralyses the hinge muscle and causes the shell to open.” Sometimes in a single night a whole bed of oysters will be totally destroyed by an invasion of starfish. Another dreaded enemy is the ‘whelk,’ a term which includes Purpura lapillus, Murex erinaceus, Buccinum undatum, and probably also Nassa reticulata. All these species perforate the shell with the end of their radula, and then suck out the contents through the neatly drilled hole. Skate fish are the cause of terrible destruction in the open beds, and a scarcely less dangerous visitant is the octopus. Crabs crush the young shells with their claws, and are said to gather in bands and scratch sand or mud over the larger specimens, which makes them open their shells. Yet another, and perhaps unconscious, foe is found in the common mussel, which takes up room meant for the young oysters, grows over the larger individuals, and harbours all sorts of refuse between and under its closely packed[112] ranks. Cliona, a parasitic sponge, bores in between the layers of the oyster’s shell, pitting them with tiny holes (corresponding to its oscula), and disturbing the inmate, who has constantly to construct new layers of shell from the inside. Weed, annelids, ‘blubber,’ shifting sand or mud, sewage or any poisoning of the water, are seriously harmful to the oyster’s best interests. A very severe winter is often the cause of wholesale destruction in the beds. According to the Daily News of 26th March 1891, the Whitstable oyster companies lost property to the value of £30,000 in the exceptionally cold winter of 1890–91, when, on the coast of Kent, the surface temperature of the sea sank below 32°, and the advancing tide pushed a small ice-floe before it. Two million oysters were laid down in one week of the following spring, to make up for the loss. During the severe winter of 1892–93 extraordinary efforts were made at Hayling I. to protect the oysters from the frost. Twenty million oysters were placed in ponds for the winter, and a steam-engine was for days employed to keep the ponds thawed and supplied with water, while large coal and coke fires were kept burning at the edge of the ponds.[226] On the other hand, the unusually warm and sunny summer of 1893 is said to have resulted in the finest fall of spat known in Whitstable for fifty years.[227]
The reproductive activity of the oyster is supposed to commence about the third year. Careful research has shown[228] that the sexes in the English oyster are not separate, but that each individual is male as well as female, producing spermatozoa as well as ova in the same gland. Here, however, two divergent views appear. Some authorities hold that the oyster does not fecundate its own eggs, but that this operation is performed by spermatozoa emitted by other specimens. It is believed that, in each individual, the spermatozoa arrive at maturity first, and that the ova are not produced until after the spermatozoa have been emitted; thus the oyster is first male and then female, morphologically hermaphrodite, but physiologically unisexual. Others are of opinion that the oyster does fecundate its own eggs, ova being first produced, and passed into the infrabranchial chamber—the ‘white-sick’ stage—and then, after an interval,[113] spermatozoa being formed and fecundating these ova—the ‘black-sick’ stage. In this latter view the oyster is first female and then male, and is, both morphologically and physiologically, hermaphrodite. The old view, that ‘black-sick’ oysters are the male, and ‘white-sick’ the female, is therefore quite incorrect.
The ova, in their earliest stage, consist of minute oval clusters of globules floating in a transparent mucus. They pass from the ovary into the gills and folds of the mantle, and are probably fecundated within the excretory ducts of the ovary, before arriving in the mantle chamber. In this stage the oyster is termed ‘white-sick.’ In about a fortnight, as the course of development proceeds, the fertilised ova become ciliated at one end (the so-called veliger stage, p. 131), and soon pigment appears in various parts of the embryos, giving them a darker colour, which varies from grayish to blue, and thus the white-sick oyster becomes ‘black-sick.’ When the black spat emerge, they are still furnished with cilia for their free-swimming life. This is of very short duration, for unless the embryo finds some suitable ground on which to affix itself within forty-eight hours, it perishes. As the spat escapes from the parent oyster, which slightly opens its valves and blows the spat out in jets, it resembles a thick cloud in the water, and is carried about at the mercy of wind and tide. April to August are the usual spawning months, warm weather being apparently an absolute necessity to secure the adhering of the spat. A temperature of 65° to 72° F. seems requisite for their proper deposit. Thus on a fine, warm day, with little wind or tide running, the spat will fall near the parents and be safely secured, while in cold, blustering weather it will certainly be carried off to a distance, and probably be altogether lost. The number of young produced by each individual has been variously estimated at from 300,000 to 60,000,000. Either extreme seems enormous, but it must be remembered that besides climatal dangers, hosts of enemies—other Mollusca, fish, and Crustacea—beset the opening career of the young oyster.
As soon as the spat has safely ‘fallen,’ it adheres to some solid object, and loses the cilia which were necessary for its swimming life. It begins to grow rapidly, increasing from about 1/20 inch in diameter to about the size of a threepenny[114] piece in five or six months, and in a year to one inch in diameter. Roughly speaking, the best guide to an oyster’s age is its size; it is as many years old as it measures inches across.
The oyster is at its prime at the age of five; its natural life is supposed to be about ten years. The rings, or ‘shoots’ on a shell are not—as is frequently supposed—marks of annual growth; cases have been noticed where as many as three ‘shoots’ were made during the year.
An oyster is furnished, on the protruding edges of the mantle, with pigmented spots which may be termed ‘visual organs,’ though they hardly rise to the capacities and organisation of real ‘eyes.’ But there is no doubt that they are sufficiently sensitive to the action of light to enable the oyster to apprehend the approach of danger, and close his doors accordingly. ‘How sensitive,’ notes Mr. W. Anderson Smith,[229] ‘the creatures are to the light above them; the shadow of the iron as it passes overhead is instantaneously noted, and snap! the lips are firmly closed.’
The geographical distribution of Ostrea edulis extends from Tränen, in Norway, close to the Arctic circle, to Gibraltar and certain parts of the Mediterranean, Holland, and N. Germany to Heligoland, and the western shores of Sleswick and Jutland. It occurs in Iceland, but does not enter the Baltic, where attempts to colonise it have always failed. Some authorities regard the Mediterranean form as a distinct species.
The literature of oyster-cookery may be passed over in silence. The curious may care to refer to M. S. Lovell’s Edible British Mollusks, where no less than thirty-nine different ways of dressing oysters are enumerated. It may, however, be worth while to add a word on the subject of poisonous oysters. Cases have been known where a particular batch of oysters has, for some reason, been fatal to those who have partaken of them. It is possible that this may have been due, in certain instances, to the presence of a superabundance of copper in the oysters, and there is no doubt that the symptoms detailed have often closely resembled those of copper poisoning. Cases of poisoning have occurred at Rochefort through’ the importation of ‘green oysters’ from Falmouth. It would no doubt be dangerous ever to eat oysters which had grown on the copper bottom of a ship. But copper is present, in more or less minute quantities, in very[115] many Mollusca, and it is more probable that a certain form of slow decomposition in some shell-fish develops an alkaloid poison which is more harmful to some people than to others, just as some people can never digest any kind of shell-fish.[230] These alkaloid developments from putrescence are called ptomaines. In confirmation of this view, reference may be made to a case, taken from an Indian Scientific Journal, in which an officer, his wife, and household ate safely of a basket of oysters for three days at almost every meal. The basket then passed out of their hands, not yet exhausted of its contents, and a man who had already eaten of these oysters at the officer’s table was afterwards poisoned by some from the same basketful.
The cultivation of the common mussel (Mytilus edulis L.) is not practised in this country, although it is used as food in the natural state of growth all round our coasts. The French appear to be the only nation who go in for extensive mussel farming. The principal of these establishments is at a little town called Esnaudes, not far from La Rochelle, and within sight of the Ile de Ré and its celebrated oyster parks. The secret of the cultivation consists in the employment of ‘bouchots,’ or tall hurdles, which are planted in the mud of the fore-shore, and upon which the mussel (la moule, as the French call it) grows. The method is said[231] to have been invented as long ago as 1235 by a shipwrecked Irishman named Walton. He used to hang a purse net to stakes, in the hope of capturing sea birds. He found, however, that the mussels which attached themselves to his stakes were a much more easily attainable source of food, and he accordingly multiplied his stakes, out of which the present ‘bouchot’ system has developed. The shore is simply a stretch of liquid mud, and the bouchots are arranged in shape like a single or double V, with the opening looking towards the sea. The fishermen, in visiting the bouchots, glide about over the mud in piroques or light, flat-bottomed boats, propelling them by shoving the mud with their feet. Each bouchot is now about 450 yards long, standing 6 feet out of the mud, making a strong wall of solid basket-work, and as there are altogether at least 500 bouchots, the total mussel-bearing length of wall is nearly 130 miles.
[116]
The mussel-spat affixes itself naturally to the bouchots nearest the sea, in January and February. Towards May the planting begins. The young mussels are scraped off these outermost bouchots, and placed in small bags made of old canvas or netting, each bag holding a good handful of the mussels. The bags are then fastened to some of the inner bouchots, and the mussels soon attach themselves by their byssus, the bag rotting and falling away. They hang in clusters, increasing rapidly in size, and at the proper time are transplanted to bouchots farther and farther up the tide level, the object being to bring the matured animal as near as possible to the land when it is time for it to be gathered. This process, which aims at keeping the mussel out of the mud, while at the same time giving it all the nutrition that comes from such a habitat, extends over about a year in the case of each individual. Quality, rather than quantity, is the aim of the Esnaudes boucholiers. The element of quantity, however, seems to come in when we are told that each yard of the bouchots is calculated to yield a cartload of mussels, value 6 francs, and that the whole annual revenue is at least £52,000.
In this country, and especially in Scotland, mussels are largely used as bait for long-line fishing. Of late years other substances have rather tended to take the place of mussels, but within the last twenty years, at Newhaven on the Firth of Forth, three and a half million mussels were required annually to supply bait for four deep-sea craft and sixteen smaller vessels. According to Ad. Meyer,[232] boughs of trees are laid down in Kiel Bay, and taken up again, after three, four, or five years, between December and March, when they are found covered with fine mussels. The boughs are then sold, just as they are, by weight, and the shell-fish sent into the interior of Germany.
Mussels are very sensitive to cold weather. In 1874, during an easterly gale, 195 acres of mussels at Boston, in Lincolnshire, were killed in a single night. They soon affix themselves to the bottom of vessels that have lain for any length of time in harbour or near the coast. The bottom of the Great Eastern steamship was at one time so thickly coated with mussels that it was estimated that a vessel of 200 tons could have been laden from her.[117] In some of our low-lying coast districts mussels are a valuable protection against inundation. “An action for trespass was brought some time ago for the purpose of establishing the right of the lord of the manor to prevent the inhabitants of Heacham from taking mussels from the sea-shore. The locality is the fore-shore of the sea, running from Lynn in a north-westerly direction towards Hunstanton in Norfolk; and the nature of the shore is such that it requires constant attention, and no little expenditure of money, to maintain its integrity, and guard against the serious danger of inundations of the sea. Beds of mussels extend for miles along the shore, attaching themselves to artificial jetties running into the sea, thereby rendering them firm, and thus acting as barriers against the sea [and as traps to catch the silt, and thus constantly raise the level of the shore]. Therefore, while it is important for the inhabitants, who claim a right by custom, to take mussels and other shell-fish from the shore, it is equally important for the lord of the manor to do his utmost to prevent these natural friends of his embankments and jetties from being removed in large quantities.”[233]
The fable that Bideford Bridge is held together by the byssi of Mytilus, which prevent the fabric from being carried away by the tide, has so often been repeated that it is perhaps worth while to give the exact state of the case, as ascertained from a Town Councillor. The mussels are supposed to be of some advantage to the bridge, consequently there is a by-law forbidding their removal, but the corporation have not, and never had, any boat or men employed in any way with regard to them.
Poisoning by mussels is much more frequent than by oysters. At Wilhelmshaven,[234] in Germany, in 1885, large numbers of persons were poisoned, and some died, from eating mussels taken from the harbour. It was found that when transferred to open water these mussels became innocuous, while, on the other hand, mussels from outside, placed in the harbour, became poisonous. The cause obviously lay in the stagnant and corrupted waters of the harbour, which were rarely freshened by tides. It was proved to demonstration that the poison was not due to decomposition; the liver of the mussels was the poisonous part. In the persons affected, the symptoms were of three[118] kinds, exanthematous (skin eruptions), choleraic, and paralytic. Cases of similar poisoning are not unfrequent in our own country, and the circumstances tend to show that, besides the danger from mussels bred in stagnant water, there is also risk in eating them when ‘out of season’ in the spawning time.
Whelks are very largely employed for bait, especially in the cod fishery. The whelk fishery in Whitstable Bay, both for bait and for human food, yields £12,000 a year. Dr. Johnston, of Berwick, estimated that about 12 million limpets were annually consumed for bait in that district alone. The cockle fishery in Carmarthen Bay employs from 500 to 600 families, and is worth £15,000 a year, that in Morecambe Bay is worth £20,000.
Cultivation of Snails for Food; Use as Medicine.—It was a certain Fulvius Hirpinus who, according to Pliny the elder,[235] first instituted snail preserves at Tarquinium, about 50 B.C. He appears to have bred several species in his ‘cochlearia,’ keeping them separate from one another. In one division were the albulae, which came from Reate; in another the ‘very big snails’ (probably H. lucorum), from Illyria; in a third the African snails, whose characteristic was their fecundity; in a fourth those from Soletum, noted for their ‘nobility.’ To increase the size of his snails, Hirpinus fed them on a fattening mixture of meal and new wine, and, says the author in a burst of enthusiasm, ‘the glory of this art was carried to such an extent that a single snail-shell was capable of holding eighty sixpenny pieces.’ Varro[236] recommends that the snaileries be surrounded by a ditch, to save the expense of a special slave to catch the runaways. Snails were not regarded by the Romans as a particular luxury. Pliny the younger reproaches[237] his friend Septicius Clarus for breaking a dinner engagement with him, at which the menu was to have been a lettuce, three snails and two eggs apiece, barley water, mead and snow, olives, beetroot, gourds and truffles, and going off somewhere else where he got oysters, scallops, and sea-urchins. In Horace’s time they were used as a gentle stimulant to the appetite, for
[119]
Escargotières, or snail-gardens, still exist in many parts of Europe, e.g. at Dijon, at Troyes and many other places in central and southern France, at Brunswick, Copenhagen, and Ulm. The markets at Paris, Marseilles, Bordeaux, Toulouse, Nantes, etc., are chiefly supplied by snails gathered from the open country, and particularly from the vineyards, in some of which Helix pomatia abounds. In the Morning Post of 8th May 1868 there is an account of the operation of clearing the celebrated Clos de Vougeot vineyard of these creatures. No less than 240 gallons were captured, at a cost in labour of over 100 francs, it being estimated that these snails would have damaged the vines to an extent represented by the value of 15 to 20 pipes of wine, against which may be set the price fetched by the snails when sold in the market.
It is generally considered dangerous to eat snails at once which have been gathered in the open country. Cases have occurred in which death by poisoning has resulted from a neglect of this precaution, since snails feed on all manner of noxious herbs. Before being sent to table at the restaurants in the great towns, they are fattened by being fed with bran in the same way as oysters.
The Roman Catholic Church permits the consumption of snails during Lent. Very large numbers are eaten in France and Austria at this time. At the village of Cauderon, near Bordeaux, it is the proper thing to end Carnival with especial gaiety, but to temper the gaiety with a dish of snails, as a foretaste of Lenten mortification.
The following species appear to be eaten in France at the present day: H. pomatia, aspersa, nemoralis, hortensis, aperta, pisana, vermiculata, lactea. According to Dr. Gray, the glassmen at Newcastle used to indulge in a snail feast once a year, and a recent writer informs us that H. aspersa is still eaten by working people in the vicinity of Pontefract and Knottingley.[239] But in this country snails appear to be seldom consciously used as an article of food; the limitation is necessary, for Lovell tells us that they are much employed in the manufacture of cream, and that a retired (!) milkman pronounced it the most successful imitation known.
Preparations made from snails used to be highly esteemed as[120] a cure for various kinds of diseases and injuries. Pliny the elder recommends them for a cough and for a stomach-ache, but it is necessary “to take an uneven number of them.”[240] Five African slugs, roasted and beaten to a powder, with half a drachm of acacia, and taken with myrtle wine, is an excellent remedy for dysentery. Treated in various ways, snails have been considered, in modern times, a cure for ague, corns, web in the eye, scorbutic affections, hectic fevers, pleurisy, asthma, obstructions, dropsy, swelling of the joints, headache, an impostume (whitlow), and burns. One of Pliny’s remedies for headache, which competes with the bones of a vulture’s head or the brain of a crow or an owl, is a plaister made of slugs with their heads cut off, which is to be applied to the forehead. He regards slugs as immature snails, whose growth is not yet complete (nondum perfectae). Lovell states that “a large trade in snails is carried on for Covent Garden market in the Lincolnshire fens, and that they are sold at 6d. per quart, being much used for consumptive patients and weakly children.”
The custom still seems to linger on in some parts of the country. Mr. E. Rundle, of the Royal Cornwall Infirmary, gives his experience in the following terms: “I well remember, some twelve years since, an individual living in an adjoining parish [near Truro] being pointed out to me as ‘a snail or slug eater.’ He was a delicate looking man, and said to be suffering from consumption. Last summer I saw this man, and asked him whether the statement that he was a ‘snail eater’ was true: he answered, ‘Yes, that he was ordered small white slugs—not snails—and that up till recently he had consumed a dozen or more every morning, and he believed they had done him good.’ There is also another use to which the country people here put snails, and that is as an eye application. I met with an instance a few weeks since, and much good seemed to have followed the use.”[241]
A reverend Canon of the Church of England, whose name I am not permitted to disclose, informs me that there was a belief among the youth of his native town (Pontypool, in Monmouthshire) that young slugs were ‘good for consumption,’ and that they were so recommended by a doctor who practised in the town. The slugs selected were about ¾ inch long, “such as[121] may be seen crawling on the turf of a hedge-bank after a shower of rain.” They were “placed upon the tongue without any previous preparation, and swallowed alive.” My informant himself indulged in this practice for some time, “not on account of any gustatory pleasure it afforded, but from some vague notion that it might do him good.”
A colleague of mine at King’s College tells me that the country people at Ponteland, near Morpeth, habitually collect Limax agrestis and boil it in milk as a prophylactic against consumption. He has himself frequently devoured them alive, but they must be swallowed, not scrunched with the teeth, or they taste somewhat bitter.
Snails have occasionally fallen, with other noxious creatures, under the ban of the Church. In a prayer of the holy martyr Trypho of Lampsacus (about 10th cent. A.D.) there is a form of exorcism given which may be used as occasion requires. It runs as follows: “O ye Caterpillars, Worms, Beetles, Locusts, Grasshoppers, Woolly-Bears, Wireworms, Longlegs, Ants, Lice, Bugs, Skippers, Cankerworms, Palmerworms, Snails, Earwigs, and all other creatures that cling to and wither the fruit of the grape and all other herbs, I charge you by the many-eyed Cherubim, and by the six-winged Seraphim, which fly round the throne, and by the holy Angels and all the Powers, etc. etc., hurt not the vines nor the land nor the fruit of the trees nor the vegetables of —— the servant of the Lord, but depart into the wild mountains, into the unfruitful woods, in which God hath given you your daily food.”
Prices given for Shells.—Very high prices have occasionally been given for individual specimens, particularly about thirty or forty years ago, when the mania for collecting was at its height. In those days certain families, such as the Volutidae, Conidae, and Cypraeidae, were the especial objects of a collector’s ardour, and he spared no expense to make his set of the favourite genus as complete as possible. Thus at Stevens’ auction-rooms in Covent Garden, on 21st July 1854, one specimen of Conus cedo nulli fetched £9: 10s., and another £16, a C. omaicus 16 guineas, C. victor £10, and C. gloria maris, the greatest prize of all, £43: 1s. At the Vernède sale, on 14th Dec. 1859 two Conus omaicus fetched £15 and £22, and a C. gloria maris £34. At the great Dennison sale, in April 1865, the Conidae fetched[122] extravagant prices, six specimens averaging over £20 apiece. Conus cedo nulli went for £18 and £22, C. omaicus for £12, C. malaccanus for 10 guineas (this and one of the cedo nulli being the actual specimens figured in Reeve’s Conchologia Iconica), C. cervus for £19 and C. gloria maris for £42. On 9th May 1866 a Cypraea Broderipii was sold at Stevens’ auction-rooms for £13, and at the Dennison sale a Cypraea princeps fetched £40, and C. guttata £42. The Volutidae, although not quite touching these prices, have yet done fairly well. Mr. Dennison’s Voluta fusiformis sold for £6: 15s., V. papillaris for £5, V. cymbiola for £5: 15s., V. reticulata for eight guineas, and two specimens of the rarest of all Volutas, V. festiva, for £14 and £16, both being figured in the Conchologia. At the same sale, two unique specimens of Oniscia Dennisoni fetched £17 and £18 respectively, and, at the Vernède sale, Ancillaria Vernèdei was bought for £6: 10s., and Voluta piperata for £7: 10s.
A unique specimen of a recent Pleurotomaria (quoyana F. and B.) was purchased by Miss de Burgh in 1873 for 25 guineas, and another species of the same genus (adansoniana Cr. and F.), of extraordinary size and beauty, is now offered for sale for about £100.
Bivalves have never fetched quite such high prices as univalves, but some of the favourite and showy genera have gone near to rival them. On 22nd June 1869, at Stevens’, Pecten solaris fetched £4: 5s., P. Reevii £4: 8s., and Cardita varia 5 guineas. Mr. Dennison’s specimens of Pecten subnodosus sold for £7, of Corbula Sowerbyi for £10, of Pholadomya candida for £8 and £13, while at the Vernède sale a Chama damicornis fetched £7.
[123]
Reproduction in the Mollusca invariably takes place by means of eggs, which, after being developed in the ovary of the female, are fertilised by the spermatozoa of the male. As a rule, the eggs are ‘laid,’ and undergo their subsequent development apart from the parent. This rule, however, has its exceptions, both among univalve and bivalve Mollusca, a certain number of which hatch their young from the egg before expelling them. Such ovoviviparous genera are Melania, Paludina, Balea, and Coeliaxis among land and fresh-water Mollusca, and Cymba and many Littorina amongst marine. The young of Melania tuberculata, in Algeria, have been noticed to return, as if for shelter, to the branchial cavity of the mother, some days after first quitting it. Isolated species among Pulmonata are known to be ovoviviparous, e.g. Patula Cooperi, P. Hemphilli, and P. rupestris, Acanthinula harpa, Microphysa vortex, Pupa cylindracea and muscorum, Clausilia ventricosa, Opeas dominicensis, Rhytida inaequalis, etc. All fresh-water Pelecypoda yet examined, except Dreissensia, are ovoviviparous.
The number of eggs varies greatly, being highest in the Pelecypoda. In Ostrea edulis it has been estimated at from 300,000 to 60,000,000; in Anodonta from 14,000 to 20,000; in Unio pictorum 200,000. The eggs of Doris are reckoned at from 80,000 to 600,000, of Loligo and Sepia at about 30,000 to 40,000. Pulmonata lay comparatively few eggs. Arion ater has been observed to lay 477 in forty-eight days (p. 42). Nests[124] of Helix aspersa have been noticed, in which the number of eggs varied from about 40 to 100. They are laid in little cup-shaped hollows at the roots of grass, with a little loose earth spread over them. The eggs of Testacella are rather large, and very elastic; if dropped on a stone floor they will rebound sharply several inches. The Cochlostyla of the Philippines lay their eggs at the tops of the great forest trees, folding a leaf together to serve as a protection.
The eggs of the great tropical Bulimus and Achatina, together with those of the Macroön group of Helix (Helicophanta, Acavus, Panda) are exceedingly large, and the number laid must be decidedly less than in the smaller Pulmonata. Bulimus oblongus, for instance, from Barbados, lays an egg about the size of a sparrow’s (Fig. 38), Achatina sinistrorsa as large as a pigeon’s. The Cingalese Helix Waltoni when first hatched is about the size of a full-grown H. hortensis. There is, in the British Museum, a specimen of the egg of a Bulimus from S. America (probably maximus or popelairanus) which measures exactly 1¾ inch in length.
The Limnaeidae deposit their eggs in irregular gelatinous masses on the under side of the leaves of water-plants, and on all kinds of débris.
Fig. 38.—Newly-hatched young and egg of Bulimus oblongus Müll., Barbados. Natural size.
The Rachiglossa or marine carnivorous families lay their eggs in tough leathery or bladdery capsules, which are frequently joined together in shapes which differ with the genus. Each capsule contains a varying number of ova. The cluster of egg-capsules of Buccinum undatum is a familiar object on all our sandy coasts. The capsules of Purpura lapillus are like delicate pink grains of rice, set on tiny stalks. They are not attached to one another, but are set closely together in groups in sheltered nooks of the rocks. A single Purpura has been observed to produce 245 capsules! Busycon lays disc-shaped capsules which are all attached at a point in the edge to a cartilaginous band nearly 3 feet in length, looking like a number of coins tied to a string at equal distances from one another. In Murex erinaceus the egg-capsules are triangular, with a short stalk.[125] They are deposited separately in clusters of from 15 to 150, there being about 20 ova in each capsule. It appears that all the species of the same genus have by no means the same method of depositing their eggs, nor do they always produce eggs of at all similar size or shape. Thus, of two British species of Nassa, N. reticulata lays egg-capsules in shape like flattened pouches with a short stalk, and fastens them in rows to the leaves of Zostera; M. incrassata, on the other hand, deposits solitary capsules, which are shaped like rounded oil-flasks. Neptunea antiqua lays its eggs in bunched capsules, like Bucc. undatum (Fig. 40), but the capsules of N. gracilis are solitary.
Fig. 39.—Various forms of spawn in Prosobranchiata: A and D, Pyrula or Busycon; B, Conus; C, Voluta musica; E, Ampullaria (from specimens in the British Museum); all × ⅔.
In Natica the eggs are deposited in what looks like a thick piece of sand-paper, curled in a spiral form (Fig. 41). The sand is agglutinated by copious mucus into a sort of sheet, and the eggs are let into this, sometimes (N. heros) in regular quincunx form. Ianthina attaches its eggs to the under side of its float (Fig. 42). The Trochidae deposit their eggs on the under side of stones and sea-weeds, each ovum being contained in a separate capsule, and all the capsules glued together into an irregular mass of varying size. The female of Galerus[126] chinensis hatches her eggs by keeping them between her foot and the stone she adheres to. They are laid in from 6 to 10 capsules, connected by a pedicle and arranged like the petals of a rose, with 10 to 12 eggs in each capsule. Those Littorina which are not ovoviviparous deposit their spawn on sea-weeds, rocks, and stones. The eggs are enveloped in a glairy mass which is just firm enough to retain its shape in the water; each egg has its own globule of jelly and is separated from the others by a very thin transparent membrane.[242]
Fig. 40.—Egg-capsules of, A, Nassa reticulata L. × ⅔; B, Buccinum undatum L. × ⅔; C, Neptunea antiqua L. × ⅓.
Fig. 41.—Spawn of a species of Natica (from a specimen in the British Museum) × ½.
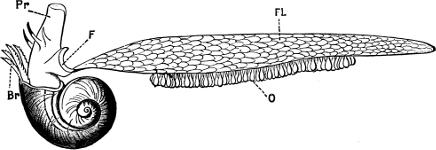
Fig. 42.—Ianthina fragilis Lam. FL, float; O, ova; Pr, proboscis; Br, branchiae; F, foot. (Quoy and Gaimard.)
Chiton marginatus, when kept in captivity, has been noticed[243][127] to elevate the posterior part of the girdle, and to pour out a continuous stream of flaky white matter like a fleecy cloud, which proved to be of a glutinous nature. It then discharged ova, at the rate of one or two every second, for at least fifteen minutes, making a total of 1300 to 1500, each being about 1/100 inch diameter. The ova were shot into the glutinous cloud, which seemed to serve as a sort of nidus to entangle the ova and prevent them being carried away. The subsequent development was rapid, and in seven days the young Chiton was hatched, being then about 1/20 inch long. Lovén has described the same species as laying its eggs, loosely united in clusters of seven to sixteen, upon small stones. There is probably some mistake about the identification, but the observation illustrates the varying methods of oviposition among allied forms.
Fig. 43.—Egg-capsules of A, Sepia elegans Orb., and B, Octopus vulgaris Lam.
Not very much is known with regard to the ovipositing of the Cephalopoda, especially those which inhabit deep water. Masses of ova arranged in very various forms have occasionally been met with floating in the ocean, but it is next to impossible to determine to what species, or even genus, they belong.[244]
In Loligo punctata the ova are contained in small cylindrical cases measuring 3 to 4 in. by ½ in., to the number of about 250 ova in each case. Hundreds of these cases are attached together like a bundle of sausages or young carrots, and the movements of the embryos within can be distinctly noted. Sepia officinalis lays large black pear-shaped capsules, each of which is tied to some place of attachment by a kind of ribbon at the upper end of the capsule, the whole forming a large group like a bunch of grapes. Octopus vulgaris deposits thousands of small berry-shaped ova, attached to a string which runs along the centre of the mass (Fig. 43).
The so-called shell of the female Argonauta is nothing more[128] than a form of protection for the ova, and is in no sense homologous to the ordinary molluscan shell. The ova consist of a large granulated mass, attached to a many branched stem; they are contained in the spire of the shell, in contact with the posterior part of the body of the mother, but sometimes project externally beyond the coil of the spire.
Certain species possess the curious property of laying their eggs on the outside of their own shells. Buccinopsis Dalei is not unfrequently found decorated with its own egg-capsules. Possibly this species, which lives on oozy ground, finds this the only secure place of attachment for its progeny. Neritina fluviatilis has a similar habit, and so have many other species of Neritina and Navicella. It is not quite clear, in the latter cases, whether the eggs are laid by the specimens on whose shell they are found, or whether they are deposited by others. In either case, perhaps the shell is the safest place for them in the rapid streams which both genera frequent. Specimens of Hydrobia ulvae taken on the wet sands at the mouth of the Dee, are found to have several little rounded excrescences scattered over the surface of the shell. These, on examination, are found to be little masses of small sand-grains, in the centre of which is a clear jelly containing segmenting ova or young embryos. Here again, in all probability, the shell is the only comparatively stable object, in the expanse of shifting sands, on which the eggs can be laid.[245]
The pulmonate genus Libera, which occurs on a few of the island groups in the Central Pacific, is remarkable for the habit of laying its eggs within its own cavernous umbilicus, which is narrowed at the lower part. The eggs number from four to six, or the same number of very young shells may be seen closely packed in the cavity, each being in shape exactly like a young Planorbis. This constriction of the umbilicus does not occur till the formation of the last two whorls, i.e. till the animal is sexually mature. Some species, but not all, provide for the safety of their eggs more completely by forming a very thin shelly plate, which nearly closes the umbilical region, and breaks away or is absorbed to facilitate the escape of the young shells.[246]
Union of Limax.—With regard to the act of union itself,[129] the method in certain species of Limax deserves special notice. L. maximus has been observed at midnight to ascend a wall or some perpendicular surface. A pair then crawl round and round one another emitting a quantity of mucus which at length forms a patch, 2 to 2½ inches in diameter. When this acquires consistency the pair begin to twist round each other in corkscrew form, and detach themselves from the wall, hanging by a cord of the thickened mucus, about 8–15 inches long, and still twisting round each other. The external generative organs are then protruded and copulation takes place, after which the bodies untwist, separate, and crawl up the cord again to the wall.[247]
Periodicity in Breeding.—In the marine Mollusca, the winter months appear to be the usual time for the deposition of eggs. Careful observations have been made on the Mollusca occurring at Naples,[248] and the general result seems to be that for all Orders alike the six winter months from November to April, roughly speaking, are the breeding time. Scarcely any forms appear to breed habitually in August, September, or October. On our own coasts, Nudibranchiata come in shore to deposit their ova from January to April. Purpura lapillus may be observed depositing ova all the year round, but is most active from January to April. Buccinum undatum breeds from October to May; Littorina all the year round.
The land Mollusca exhibit rather more periodicity than the marine. In temperate climates they breed exclusively in the summer months. In the tropics their periods are determined by the dry and rainy seasons, where such occur, otherwise they cohabit all the year round. According to Karl Semper, the snails of the warm Mediterranean region arrive at sexual maturity when they are six months old, i.e. before they are fully grown. After a rest of about three months during the heat of summer, a second period of ovipositing occurs.[249] Helix hortensis and H. nemoralis ascend trees, sometimes to a height of forty feet, when pairing.[250]
Hybridism as the result of union between different species of Mollusca is exceedingly rare. Lecoq once noticed[251] on a wall at Anduze (Gard) as many as twenty specimens of Pupa cinerea[130] united with Clausilia papillaris. No offspring seem to have resulted from what the professor calls ‘this innocent error,’ for the wall was carefully scrutinised for a long time, and no hybrid forms were ever detected.
The same observer noticed, in the Luxembourg garden at Paris, and M. Gassies has noticed[252] at various occasions, union between Helix aspersa and nemoralis, H. aspersa and vermiculata, between Stenogyra decollata and a Helix (sp. not mentioned), H. variabilis and pisana, H. nemoralis and hortensis. In the two latter cases a hybrid progeny was the result. It has been noticed that these unions generally took place when the air was in a very electric condition, and rain had fallen, or was about to fall, abundantly.
Of marine species Littorina rudis has been noticed[253] in union both with L. obtusata and with L. littorea, but no definite facts are known as to the result of such unions.
Self-impregnation (see p. 44).
Development of the Fertilised Ovum.—The first stages in the development of the Mollusca are identical with those which occur in other classes of animals. The fertilised ovum consists of a vitellus or yolk, which is surrounded with albumen, and is either contained in a separate capsule, or else several, sometimes many, ova are found in the same capsule, only a small proportion of which ultimately develop. The germinal vesicle, which is situated at one side of the vitellus, undergoes unequal segmentation, the result of which is usually the formation of a layer of small ectoderm cells overlying a few much larger cells which contain nearly the whole of the yolk. The large cells are then invaginated, or are simply covered by the growth of the ectoderm cells. The result in either case is the formation of an area, the blastopore, where the inner cells are not covered by the ectoderm. The blastopore gradually narrows to a circular opening, which, in the great majority of cases, eventually becomes the mouth. The usual differentiation of germinal layers takes place, the epiblast eventually giving rise to the epidermis, nervous system, and special sense organs, the hypoblast to the liver and to the middle region of the alimentary tract, the mesoblast to the muscles, the body cavity, the vascular, the excretory and reproductive[131] systems. The next, or trochosphere (trochophora) stage, involves the formation of a circlet of praeoral cilia, dividing the still nearly spherical embryo into two unequal portions, the smaller of which consists simply of the prostomium, or part in front of the mouth, the larger bearing the mouth and anus.
So far the series of changes undergone by the embryo are not peculiar to the Mollusca; we now come to those which are definitely characteristic of that group. The stage next succeeding the development of the trochosphere is the definitive formation of the velum, a process especially characteristic of the Gasteropoda and Pelecypoda, but apparently not occurring in the great majority of land Pulmonata.
Fig. 44.—Veligers of Dentalium entalis L.: A, longitudinal section of a larva 14 hours old, × 285; B, larva of 37 hours, × 165; C, longitudinal section of larva of 34 hours, × 165; m, mouth; v, v, velum. (After Kowalewsky.)
The circlet of cilia becomes pushed more and more towards the anterior portion of the embryo, the cilia themselves become longer, while the portion of the body from which they spring becomes elevated into a ridge or ring, which, as a rule, develops on each side a more or less pronounced lobe. The name velum is applied to this entire process of ciliated ring and lobes, and to the area which they enclose.
Fig.45.—Veliger of Patella vulgata L., 130 hours old: f, rudimentary foot; op, operculum; sh, shell; v, v, velum. (After Patten, highly magnified.)
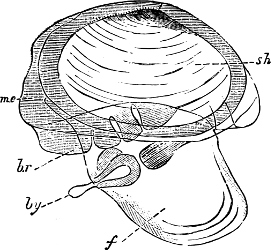
Fig. 46.—Developed larva of Cyclas cornea L.: br, rudimentary branchiae; by, byssus; f, foot; m.e, mantle edge; sh, shell. (After Ziegler, highly magnified.)
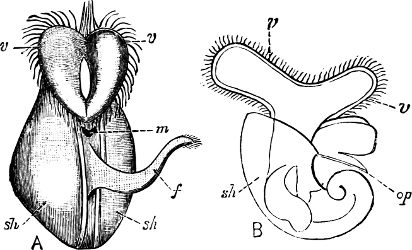
Fig. 47.—A, Advanced veliger of Dreissensia: f, foot; m, mouth; sh, shell; v, v, velum. (After Korschelt and Heider, much enlarged.) B, Veliger of a Pteropod (Tiedemannia): op, operculum; sh, shell; v, velum. (After Krohn, much enlarged.)
In this so-called veliger stage, the velum serves, in the first place, to cause rotation of the larva within the egg-capsules, and, after hatching, as an organ of locomotion. As a rule, the velum disappears entirely in the adult mollusc after the free-swimming stage is over, but in the common Limnaea stagnalis it persists, losing its cilia, as the very prominent circum-oral lobes. Simultaneously with the development of the velum, and in some cases[132] earlier, appear the rudiments of the shell-gland and of the foot, the latter being situated on the ventral side, between the mouth and anus, the former on the dorsal side, behind the velum, and above the surface of the eventual visceral sac. Thus the prime characteristics of the veliger stage, subsequent to the appearance of the velum itself, are the development of the visceral sac and shell-gland on the upper, and of the foot on the under side. According to Lankester the primitive shell-gland does not, as a rule, directly give rise to the shell of the adult mollusc, but becomes filled up by a horny substance, and eventually disappears; the permanent shell then forms over the surface of the visceral hump from the original centre of the shell-gland. It is[133] only in Chiton, and possibly in Limax, that the primitive shell-sac is retained and developed into the final shell-forming area, which is much wider, and extends to the edges of the mantle. Within the velar area first appear the rudiments of the tentacles and eyes; when these become developed the velum atrophies and disappears.
Several of these veligers when captured in the open sea have been mistaken for perfect forms, and have been described as such. Thus the larva of Dolium has been described as Macgillivrayia, that of a Purpura as Chelotropis and Sinusigera, that of Aporrhais pes pelecani as Chiropteron, that of Marsenia conspicua as Brownia, Echinospira, and Calcarella.
Cephalopoda.—The embryonic development of the Cephalopoda is entirely distinct from that of all other Mollusca. The segmentation of the vitellus is partial, and the embryo is furnished with a vitelline sac, which is very large in the majority of cases (Fig. 48). There is no free-swimming stage, but the embryo emerges from the egg fully developed.
Differences of Sex.—In the Mollusca there are two main types of sexual difference: (i) sexes separate (dioecious type), (ii) sexes united in the same individual (hermaphrodite type).
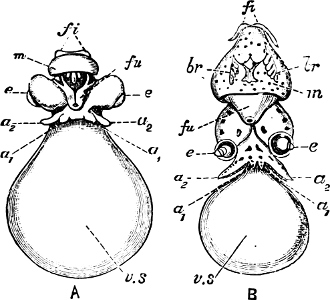
Fig. 48.—Two stages in the development of Loligo vulgaris Lam.: a1, a1, first, and a2, a2, second pairs of arms; br, branchiae, seen through m, mantle; e, e, eyes; fi, fins; fu, funnel; v.s, vitelline sac. (After Kowalewsky.)
In some cases—e.g. certain Pelecypoda—what is practically a third type occurs. The animal is hermaphrodite, but the male and female elements are not developed simultaneously, i.e. the same individual is at one time female, at another male.
1. The sexes are separate in
[134]
2. The sexes are united in
In the dioecious Mollusca, sexual union is the rule, but is by no means universal. In some instances,—e.g. Vermetus, Magilus, Patella, Haliotis, Crepidula, Chiton, the Scaphopoda—the form and habits of the animal do not admit of it; in others (many Trochus) a male copulative organ is wanting. When this is the case, the male scatters the spermatozoa freely; the majority must perish, but some will be carried by currents in the direction of the female.
When the sexes are separate, the female is frequently larger than the male. This is markedly the case in Littorina, Buccinum, and all the Cephalopoda; in Argonauta the difference is extreme, the male not being more than ¼ the size of the female.
Those hermaphrodite Mollusca which are capable of sexual union (Gasteropoda, Pulmonata, and Opisthobranchiata) are conveniently divided into two sections, according as (1) there are separate orifices for the male and female organs, or (2) one orifice serves for both. To the former section (Digonopora[2]) belong the Limnaeidae, Vaginulidae, and Onchidiidae, and many Opisthobranchiata, including all the Pteropoda; to the latter (Monogonopora[255]) nearly all the Nudibranchiate Opisthobranchiata, and all the rest of the Pulmonata. In the latter case during union, mutual impregnation takes place, and each of the two individuals concerned has been observed (compare p. 42) to deposit eggs. In the former, however, no such reciprocal act can take place, but the same individual can play the part of male to one and female to another, and we sometimes find a string of Limnaea thus united, each being at once male and female to its two adjacent neighbours.
The Reproductive System.—Broadly speaking, the complicated arrangements which are found in Mollusca resolve themselves into modifications of three important factors:—
(a) The gonads or germ-glands, in which are developed the[135] ova and the spermatozoa. These glands are generally known as the ovary in the female, the sperm-gland or testis in the male.
(b) The channels which provide for the passage of the seminal products; namely, the oviduct in the female, the vas deferens or sperm-duct in the male.
(c) The external generative organs.
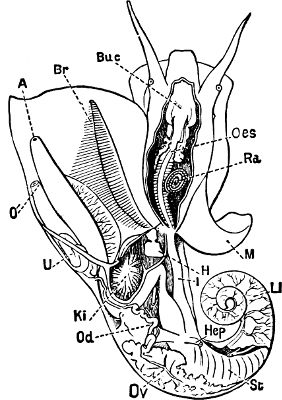
Fig. 49.—Generative and other organs of Littorina obtusata L., female.
(After Souleyet.)
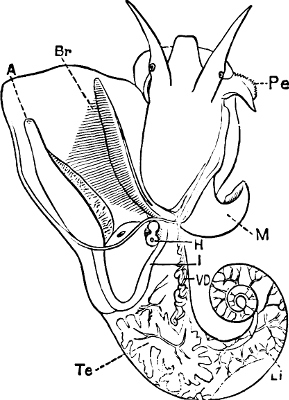
Fig. 50.—Generative and other organs of Littorina obtusata L., male.
(After Souleyet.)
Dioecious Mollusca.—The common Littorina obtusata will serve as a typical instance of a dioecious prosobranchiate, exhibiting the simplest form of organs. In the female the ovary, a lobe-shaped body, is embedded in the liver. An oviduct with many convolutions conveys the ova into the uterus, an oblong chamber which consists simply of a dilatation of the oviduct. The ova descend into the uterus, which is sometimes furnished with a seminal pouch. In this seminal pouch, or above it, in the oviduct, the ova come into contact with the spermatozoa. The lower part of the uterus secretes a gelatinous medium (or[136] capsule, as the case may be) in which the fertilised ova become enclosed previous to exclusion. In position the oviduct abuts on the kidney, while the uterus is in close proximity to the rectum, and the female external orifice is found close to the anus, within the branchial cavity.
The male organs of Littorina are more simple. The testis is lodged, like the ovary, in the liver; the vas deferens is, like the oviduct, convoluted, and eventually traverses the right side of the neck, emerging near the right tentacle, and terminating in the penis or external copulative organ (Fig. 50).
This system prevails, with but slight modifications in detail, throughout the prosobranchiate Gasteropoda. The most important modification is the passage of the seminal products in certain cases (many of the Diotocardia) through the right kidney, with which the oviduct and vas deferens always stand in close relation. The same arrangement occurs in the Scaphoda and some Pelecypoda.
The penis varies greatly in form and size. In the Strombidae (see Fig. 99) and Buccinidae (Fig. 62) it is very large and prominent; in Littorina it is somewhat spinulose at one side; in Paludina a portion of it is lodged in the right tentacle, which becomes atrophied and much more obtuse than the tentacle on the left side.
Spermatozoa.—The shape of the spermatozoa and of the ova in Mollusca is of the usual type. In Paludina Ampullaria, and certain species of Murex two types of spermatozoa occur, one hair-like, the other worm-like, three times as long as the former, and not tapering at one end. The former type alone take part in fertilisation, and penetrate the ovum. It has been suggested that these worm-like spermatozoa are a kind of incipient ova, and indicate a possible stage in commencing hermaphroditism. And, since the nearest allies of the Prosobranchiata (in which these types occur) are hermaphrodite (i.e. the Opisthobranchiata and Pulmonata), it is not unreasonable to suppose that the Prosobranchiata should show some tendency towards hermaphroditism in their genital glands.[256]
Cephalopoda.—The special characteristic of the reproductive organs in female Cephalopoda is the development of various glands, some of considerable size, in connexion with the ovary and oviduct. Sepia, Loligo, and Sepiola are furnished with two[137] large nidamental glands, which open into the mantle cavity independently of the oviduct. Their purpose is to produce a viscid mucus, which envelops the ova at the moment of their emission and eventually hardens into the egg-capsules. A pair of accessory nidamental glands occur in Sepia, as well as a pair of smaller glands situated on the oviduct itself.
In many of the male Cephalopoda the vas deferens is long and dilated at its outer end into a glandular reservoir, within which are formed the spermatophores, or narrow cylindrical packets which contain a very large number of spermatozoa. When charged, the spermatophores pass into what is known as Needham’s sac, where they remain until required for use. These spermatophores are a very characteristic part of the reproductive arrangements in the Cephalopoda. The male of Sepia has been noticed to deposit them, during union, upon the buccal membrane of the female. During the emission of the ova by the female, the spermatophores, apparently through the agency of a kind of spring contained at one end, burst, and scatter the spermatozoa over the ova.
The Hectocotylus Arm.—Perhaps the most remarkable feature in the sexual relations of all the Mollusca is the so-called hectocotylus of the Cephalopoda. In the great majority of the male Cephalopoda, one of the ‘arms,’ which is modified for the purpose in various ways and to a greater or less extent, becomes charged with spermatophores, and sometimes, during union, becomes detached and remains within the mantle of the female, preserving for some considerable time its power of movement.
The hectocotylus is confined to the dibranchiate Cephalopoda, and its typical form, i.e. when part of the arm becomes disengaged and left with the female, occurs only in three genera of the Octopodidae, viz.[Argonauta, Ocythoe (Philonexis), and Tremoctopus. In all of these, the male is many sizes smaller than the female. In Argonauta the third arm on the left side becomes hectocotylised. At first it is entirely enveloped in a kind of cyst, in such a way that only a small portion of the tip projects; subsequently the cyst parts asunder, and allows the arm to become expanded to its full length, which considerably exceeds that of the other arms. At a certain point the acetabula or suckers terminate, and the remainder of the arm consists of a very long, tapering, sometimes thread-like filament, which is[138] pointed at the extreme tip. It is not yet known how the spermatophores find their way into the hectocotylus, or how the hectocotylus impregnates the ova of the female. The arm thus affected is not always the same. In Tremoctopus it is the third of the right side, in the Decapoda the modification usually affects the fourth of the left.
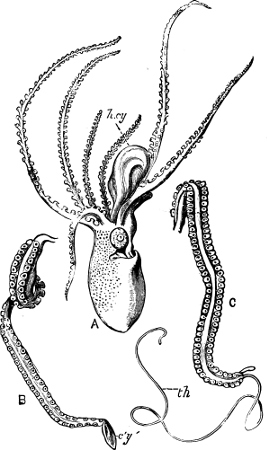
Fig. 51.—Male of Ocythoe tuberculata Raf. (= Philonexis catenulatus Fér.), Mediterranean, showing three stages, A, B, and C, in the development of the hectocotylus arm: h.cy, hectocotylus still in the cyst; c´y´, spoon-shaped cyst at the end of the arm when freed; th, thread-like organ freed by the rupture of c´y´. Natural size. From specimens in the British Museum.
This singular property of the male Cephalopoda has only recently been satisfactorily explained. It is true that Aristotle, more than twenty-two centuries ago, distinctly stated that certain of the arms were modified for sexual purposes. Speaking of what he calls the polypus (which appears to represent the Octopus vulgaris of the Mediterranean), he says: ‘It differs from the female in having what the fishermen call the white sexual organ on its arm;’ again, ‘Some say that the male has something of a sexual nature (αὶδοιῶδές τι) on one of its arms, that on[139] which the largest suckers occur; that this is a kind of muscular appendage attached to the middle of the arm, and that it is entirely introduced within the funnel of the female’. Unfortunately the word translated by introduced is corrupt, and can only be restored conjecturally. He again remarks, ‘The last of the arms, which tapers to a fine point and is the only whitish arm, it uses in sexual union.’[257]
The typical hectocotylus seems to have entirely escaped notice until early in the present century, when both Delle Chiaje and Cuvier described it, as detected within the female, as a parasite, the latter under the name of Hectocotylus octopodis. Kölliker, in 1845–49, regarded the Hectocotylus of Tremoctopus as the entire male animal, and went so far as to discern in it an intestine, heart, and reproductive system. It was not until 1851 that the investigations of Vérany and Filippi confirmed a suggestion of Dujardin,[258] while H. Müller, in 1853, completed the discovery by describing the entire male of Argonauta.
In all genera of dibranchiate Cephalopoda except Argonauta, Ocythoe, and Tremoctopus, one of the arms is sexually modified in various ways, but never becomes so much prolonged, and is never detached and left with the female. In Loligo Forbesii Stp. the fourth arm on the left has 23 pairs of regularly developed acetabula, which then lessen in size and disappear, being replaced by long pedunculated papillae, of which there are about 40 pairs. In Loligo vulgaris Lam. and L. Pleii Orb. 18 or 19 pairs of acetabula are regularly formed, and then occur 40 pairs of papillae, as in Forbesii. In other species of Loligo (gahi Orb., brevis Bl., brasiliensis Orb.) only the outer row of suckers becomes modified into papillae after about the 20th to the 22nd pair. In Sepioteuthis sepioides the modification is the same as in the Loligo last mentioned, but the corresponding arm on the right side is so covered with acetabula towards its extreme end, that it is thought that it in some way co-operates with the hectocotylised left arm.
In Octopus, the third arm on the right side is subject to modification. This arm is always shorter than the corresponding arm on the other side, and carries fewer suckers, but is furnished[140] at the extreme tip with a peculiar kind of plate, which connects with the membrane at the base of the arm by a channel of skin, which probably conveys the spermatophores up to the tip.
In Octopus vulgaris, the species referred to by Aristotle, the hectocotylised arm is short, thin in its outer half and pointed at the extremity, while the fold of skin is very white, and gives the arm an appearance of being divided by a cleft at the side. At the same time, an unusual development of one or two suckers on the arm is not uncommon.[259]
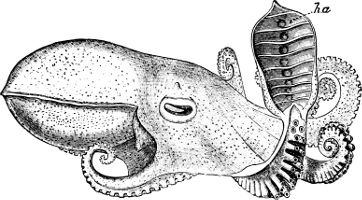
Fig. 52.—Octopus lentus Baird, N. Atlantic, showing the peculiar formation of the hectocotylus arm, h.a. (After Verrill, × ½.)
It is believed that in the Tetrabranchiate Cephalopoda (Nautilus) a union of the four inner ventral arms may correspond functionally to the hectocotylising of the arm in the Dibranchiates.
Hermaphrodite Mollusca.—(a) Monogonopora.—The reproductive system in the hermaphrodite Mollusca is far more complicated than in the dioecious, from the union of the male and female organs in the same individual. As a type of the Monogonopora, in which a single orifice serves for both male and female organs, may be taken the common garden snail (Helix aspersa), the accompanying figure of which is drawn from two specimens found in the act of union (Fig. 53).
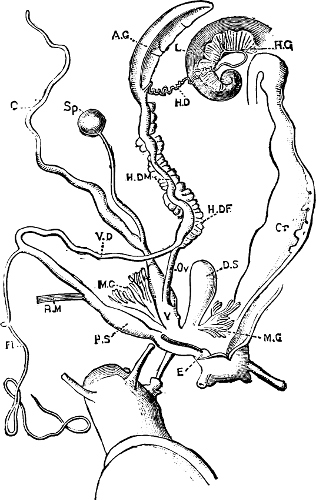
Fig. 53.—Genitalia of Helix aspersa Müller, drawn from two individuals in the act of union, from a dissection by F. B. Stead.
Beginning from the inside and proceeding outwards we have firstly the hermaphrodite gland or ovo-testis (H.G.), a yellowish white mass of irregular shape, embedded in the liver (L.) and forming part of its spiral but not reaching quite to the apex. Within this gland are developed the ova and spermatozoa. The former are rather large round cells, produced within the outer[141] wall of the gland, while the spermatozoa, which are produced in the more central part, are thread-like bodies, generally aggregated in small bundles. From the hermaphrodite gland the ova and spermatozoa pass through the upper part of the hermaphrodite duct (H.D.), which is always more or less convoluted. Below the convoluted portion, the duct opens into the albumen gland (A.G.), a large linguiform mass of tissue which becomes dilated at the time of pairing, and secretes a thick viscid fluid which probably serves to envelop the ova. Up to this point both the male and female elements follow the same course, but on their exit from the albumen gland they diverge. The hermaphrodite duct becomes greatly enlarged, and is partially divided by a kind of septum into a male and female portion. These run parallel to one another, the larger or female portion (H.DF.), through which the ova pass (and which is sometimes termed the uterus) being dilated into a number of puckered folds, while the smaller or male portion (H.DM.) is comparatively narrow, and not dilated. At their anterior end, the two portions of the[142] duct separate completely from one another, the female portion being then termed the oviduct (OV.) and the male portion the vas deferens (V.D.).
Following first the oviduct, we find that it soon widens into the vagina (V.), which is furnished with a pair of mucous glands (M.G.), one on each side. These are much branched, and resemble little bunches of whitish seaweed. A little above the mucous glands a long tube diverges from the vagina, which is furnished with a produced coecum (C.) and a pouch, the spermatheca (SP.) at the extreme end. In this pouch, and in the duct leading to it, is stored the spermatophore received in union with another snail. Just below the mucous glands the vagina is joined by the dart sac (D.S.), which is more fully described below. Finally, at its lower end the vagina unites with the penis sac at a point just posterior to the common orifice.
Returning now to the male organs, we find that the vas deferens is the continuation of the male portion of the hermaphrodite duct, after its final separation from the female portion. It passes under the retractor muscle of the upper right tentacle, which has been cut away in the specimen figured, to dissect it out. Just before the vas deferens widens into the penis sac, it branches off into a long and tapering tube, the flagellum, in which the spermatozoa are stored and become massed together in the long packet known as the spermatophore. The penis sac (P.S.) is the continuation of the vas deferens beyond the point at which the flagellum diverges. It joins the vagina at its extreme anterior end, uniting with it to form the common genital aperture, which cannot be exactly represented in the figure. The penis itself lies in the interior of the penis sac, and is a rather long muscular tube which is protruded during union, but at other times remains retracted within the sac.
In the Helicidae generally, the form of the generative organs varies with each separate species, sometimes merely as regards the size of the different parts, at others in the direction of greater simplicity or complication. The mucous glands may be absent, and the flagellum greatly reduced in size, or absent altogether.
The Dart Sac.—A remarkable part of the reproductive system in many of the true Helicidae is the so-called dart, Liebespfeil, or telum veneris. It consists of ‘a straight, or curved, sometimes slightly twisted tubular shaft of carbonate of lime,[143] tapering to a fine point above, and enlarging gradually, more often somewhat abruptly, to the base.’ The sides of the shaft are sometimes furnished with two or more blades; these are apparently not for cutting purposes, but simply to brace the stem. The dart is contained in a dart sac, which is attached as a sort of pocket to the vagina, at no great distance from its orifice. There are four different forms of sac. It may be single or double, and each of these divisions may be bilobed, each lobe containing one dart at a time. In Helix aspersa the dart is about 5/16 in. in length, and ⅛ in. in breadth at its base (see Fig. 54).
It appears most probable that the dart is employed as an adjunct to the sexual act. Besides the fact of the position of the dart sac anatomically, we find that the darts are extruded and become embedded in the flesh just before or during the act of copulation. It may be regarded, then, as an organ whose punctures induce excitement preparatory to sexual union. It only occurs in well-grown specimens. When once it begins to form, it grows very rapidly, perhaps not more than a week being required for its entire formation.
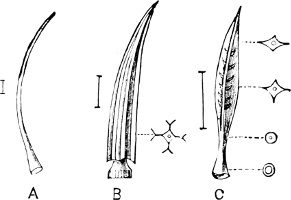
Fig. 54.—Darts of British land snails: A, Hyalinia excavata Bean; B, Helix hortensis Müll.; C, Helix aspersa Müll. (After Ashford.)
The dart is almost confined to Helicidae, a certain number of exceptions being known which border on Helix. Hyalinia nitida and excavata are the only British species, not Helices, which are known to possess it. It has not been noticed to occur in the slugs, except in the N. American genus Tebennophorus. About one-third of the British Helices are destitute of the dart.[260] H. rufescens possesses a double bilobed sac, but only two darts, which lie in the lower lobes. It does not use the darts, and could not do so, from the relative sizes of dart and sac; it has often been watched when uniting, but the use of the darts has never been observed. From this it has been inferred that the darts are degenerate weapons of defence, and that they were in fact at one time much stronger organs and more often used.[261] This theory, however, does not seem consistent with[144] the whole circumstances of the occurrence, position, and present use of the darts.
Hermaphrodite Mollusca.—(b) Digonopora.—As an example of the Digonopora or hermaphrodite Mollusca with separate generative apertures for the male and female organs, we may take the common Limnaea stagnalis (Fig. 55). It will be seen from the figure that the relative positions of the hermaphrodite gland and duct, and of the albumen gland, are the same as in Helix. When the oviduct parts company from the vas deferens, it becomes furnished with several accessory glands, one of which (Gl.E.) probably serves as a reservoir for the ova, and answers more or less to a uterus. The tube leading to the spermatheca is short, and there is no divergent caecum. The female orifice lies near to the external opening of the branchial cavity. The vas deferens, which is very long, is furnished with a large prostate gland. The penis sac is greatly dilated, and there is no flagellum. The male orifice is behind the right tentacle, slightly in advance of the female orifice (compare Fig. 102).
Fig. 55.—Genitalia of Limnaea stagnalis L. (from a dissection by F. B. Stead), × 2.
Most of the Opisthobranchiata, but not all, have separate sexual orifices. Numerous variations from the type just described[145] will be found to occur, particularly in the direction of the development of accessory glands, which are sometimes very large, and whose precise purpose has in many cases not been satisfactorily determined.
Pelecypoda.—In the dioecious Pelecypoda, which form the great majority, the reproductive system is simple, and closely parallel in both sexes. It consists of a pair of gonads, which are either ovaries or testes, and a pair of oviducts or sperm-ducts which lead to a genital aperture. The gonads are usually placed symmetrically at the sides or base of the visceral mass. The oviduct is short, and the genital aperture is usually within the branchial chamber, thus securing the fertilisation of the ova by the spermatozoa, which are carried into the branchial chamber with the water which passes through the afferent siphon.
Hermaphrodite Pelecypoda are rare, the sexes being usually separate. The following are assured instances: Pecten glaber, P. jacobaeus, P. maximus, Ostrea edulis, Cardium norvegicum, Pisidium pusillum, Cyclas cornea, Pandora rostrata, Aspergillum dichotomum, and perhaps Clavagella. The greater number of these have only a single genital gland (gonad) on each side, with a single efferent duct from each, but part of the gland is male and part female, e.g. in the Pectens above mentioned. Pandora and Aspergillum have two distinct glands, respectively male and female, on each side, each of the two glands possessing its separate duct, and the two ducts from each side eventually opening near one another. It appears probable that the Septibranchiata (Cuspidaria, Poromya, Lyonsiella, etc.) must also be added to the number of hermaphrodite Pelecypoda which have separate male and female glands.
It is worthy of remark that all the hermaphrodite Pelecypoda belong to forms decidedly specialised, while forms distinctly primitive, such as Nucula, Solenomya, Arca, and Trigonia are all dioecious. In Gasteropoda similarly, the least specialised forms (the Amphineura, with the exception of the Neomeniidae, and the Rhipidoglossa) are dioecious. It is possible therefore that in the ancestors of the Mollusca the separation of the sexes had already become the normal type of things, and that hermaphroditism in the group is, to a certain extent, a sign or accompaniment of specialisation.[262]
[146]
Development of Fresh-water Bivalves.—The vast majority of fresh-water bivalves either pass the larval stage entirely within the mother, and do not quit her except in a perfectly developed form (Cyclas, Pisidium), or assume a mode of development in which free larvae indeed occur, but are specially modified for adaptation to special circumstances (Unio). Cyclas and Pisidium, and no doubt all the kindred genera, preserve their ova in a sort of brood-pouch within the gills, in which the ova pass the earlier stages of their development. But, even so, the larva of these genera retains some traces of its original free-swimming habits, for a rudimentary velum, which is quite useless for its present form of development, has been detected in Cyclas.
The larva of Dreissensia (see Fig. 47, A), so far as is at present known, stands alone among fresh-water bivalves in being free-swimming, and to this property has been attributed, no doubt with perfect justice, the fact of the extraordinarily rapid spread of Dreissensia over the continent of Europe (chap. xvi.). In expelling the ova, the parent slightly opens the shells and then quickly closes them, shooting out a small point of white slime, which is in fact a little ball of eggs. The general course of development is precisely parallel to that of marine Pelecypoda, greatly resembling, so far as form is concerned, certain stages in the growth of the larvae of Modiolaria and Cardium, as figured by Lovén.[263]
In June and July the larvae appear in large numbers on the surface of the water, when in spite of their exceedingly small size, they can be captured with a fine hand-net. They pass about eight days on the surface, feeding apparently on minute floating algae. During this time, the principal change they undergo is in the formation of the foot, which first appears as a small prominence midway between the mouth and anus, and gradually increases in length and flexibility. When the larva sinks to the bottom, the velum soon disappears entirely, the foot becomes exceedingly long and narrow, while the shell is circular, strongly resembling a very young Cyclas.
Larvae of Unionidae.—The early stages of the development of Unio and Anodonta (so far as the species of North America, Europe, and Asia are concerned) is of extreme interest, from the remarkable fact that the young live for some time[147] parasitically attached to certain species of fresh-water fishes. In order to secure this attachment, the larva, which is generally known as Glochidium, develops a long filament which perhaps renders it aware of the neighbourhood of a fish, and also a larval shell furnished with strong hooks by which it fastens itself to the body of its unconscious host (Fig. 56). According to some interesting observations made by Mr. O. H. Latter,[264] the ova pass into the external gill of the mother, in which is secreted a nutritive mucus on which they are sustained until they arrive at maturity and a suitable opportunity occurs for their ‘being born.’ If this opportunity is deferred, and the Glochidia mature, their so-called ‘byssus’ becomes developed, and by being entangled in the gill filaments of the parent, prevents their escaping. It is interesting to notice that, when the nutritive mucus of the parent is used up, it becomes, as it were, the turn of the children to provide for themselves a secondary mode of attachment.
Fig. 56.—A, Glochidium immediately after it is hatched: ad, adductor muscle; by, ‘byssus’ cord; s, sense organs; sh, shell. B, Glochidium after it has been on the fish for some weeks: a.ad, p.ad, anterior and posterior adductors; al, alimentary canal; au.v, auditory vesicle; br, branchiae; f, foot; mt, mantle. (Balfour.)
The mother Anodonta does not always retain the Glochidium until fish are in her neighbourhood. Gentle stirring of the water caused them to emit Glochidium in large masses, if the movement was not so violent as to cause alarm. The long slimy masses of Glochidium were observed to be drawn back again within the shell of the mother, even after they had been ejected to a distance of 2 or 3 inches.
It is a mistake to assert that the young Glochidium can swim. When they finally quit the mother, they sink to the[148] bottom, and there remain resting on their dorsal side, with the valves gaping upwards and the so-called byssus streaming up into the water above them. There they remain, until a convenient ‘host’ comes within reach, and if no ‘host’ comes within a certain time, they perish. They are evidently peculiarly sensitive to the presence of fish, but whether they perceive them by smell or some other sense is unknown. “The tail of a recently killed stickleback thrust into a watch-glass containing Glochidium throws them all into the wildest agitation for a few seconds; the valves are violently closed and again opened with astonishing rapidity for 15–25 seconds, and then the animals appear exhausted and lie placid with widely gaping shells—unless they chance to have closed upon any object in the water (e.g. another Glochidium), in which case the valves remain firmly closed.”
In about four weeks after the Glochidium has quitted its host, and the permanent shell has made its appearance within the two valves of the Glochidium, the projecting teeth of the latter press upon the ventral edge of the permanent shell, at a point about half way in its lengthward measurement, retarding the growth of the shell at that particular point, and indenting its otherwise uninterrupted curve with an irregular notch or dent. As growth proceeds, this dent becomes less and less perceptible on the ventral margin of the shell itself, but its effects may be detected, in well-preserved specimens, by the wavy turn in the lines of growth, especially near the umbones of the young shell.
Mr. Latter found that all species of fish with which he experimented had a strong dislike to Glochidium as an article of food. Sometimes a fish would taste it “just to try,” but invariably spit it out again in a very decided manner. The cause of unpleasantness seemed not to be the irritation produced in the mouth of the fish by the attempt of the Glochidium to attach itself, but was more probably due to what the fish considered a nasty taste or odour in the object of his attentions.
The following works will be found useful for further study of this portion of the subject:—
F. M. Balfour, Comparative Embryology, vol. i. pp. 186–241.
F. Blochmann, Ueber die Entwickelung von Neritina fluviatilis Müll.: Zeit. wiss. Zool. xxxvi. (1881), pp. 125–174.
L. Boutan, Recherches sur l’anatomie et le développement de la Fissurelle: Arch. Zool. exp. gén. (2) iii. suppl. (1885), 173 pp.
[149]
W. K. Brooks, The development of the Squid (Loligo Pealii Les.): Anniv. Mem. Bost. Soc. Nat. Hist. 1880.
„ „ The development of the oyster: Studies Biol. Lab. Johns Hopk. Univ. i. (1880), 80 pp.
R. von Erlanger, Zur Entwickelung von Paludina vivipara: Morph. Jahrb. xvii. (1891), pp. 337–379, 636–680.
„ „ Zur Entwickelung von Bythinia tentaculata: Mitth. Zool. Stat. Neap, x. (1892), pp. 376–406.
H. Fol, Sur le développement des Ptéropodes: Arch. Zool. exp. gén. iv. (1875), pp. 1–214.
„ Etudes sur le développement des Mollusques. Hétéropodes: ibid v. (1876), pp. 105–158.
„ Etudes sur le développement des Gastéropodes pulmonés: ibid. viii. (1880), pp. 103–232.
H. Grenacher, Zur Entwickelungsgeschichte der Cephalopoden: Zeit. wiss. Zool. xxiv. (1874), pp. 419–498.
B. Hatschek, Ueber Entwickelungsgeschichte von Teredo: Arb. Zool. Inst. Univ. Wien, iii. (1881), pp. 1–44.
R. Horst, On the development of the European oyster: Quart. Journ. Micr. Sc. xxii. (1882), pp. 339–346.
E. Korschelt and K. Heider, Lehrbuch der vergleichenden Entwickelungsgeschichte der wirbellosen Thiere, Heft iii. (1893), pp. 909–1177 (the work is in process of translation into English).
A. Kowalewsky, Embryogénie du Chiton polii avec quelques remarques sur le développement des autres Chitons: Ann. Mus. Hist. Nat. Mars. Zool. i. (1883), v.
E. Ray Lankester, Contributions to the developmental history of the Mollusca: Phil. Trans. Roy. Soc. vol. 165 (1875), pp. 1–31.
„ „ Observations on the development of the pond-snail (Lymnaeus stagnalis), and on the early stages of other Mollusca: Quart. Journ. Micr. Sc. xiv. (1874), pp. 365–391.
„ „ Observations on the development of the Cephalopoda: ibid. xv. (1875), pp. 37–47.
W. Patten, The embryology of Patella: Arb. Zool. Inst. Univ. Wien, vi. (1886), pp. 149–174.
M. Salensky, Études sur le développement du Vermet: Arch. Biol. vi. (1885), pp. 655–759.
L. Vialleton, Recherches sur les premières phases du développement de la Seiche (Sepia officinalis): Ann. Sc. Nat. Zool. (7) vi. (1888), pp. 165–280.
S. Watase, Observations on the development of Cephalopods: Stud. Biol. Lab. Johns Hopk. Univ. iv. (1888), pp. 163–183.
„ „ Studies on Cephalopods: Journ. Morph. iv. (1891), pp. 247–294.
E. Ziegler, Die Entwickelung von Cyclas cornea Lam.: Zeit. wiss. Zool. xli. (1885), pp. 525–569.
[150]
The principle of respiration is the same in the Mollusca as in all other animals. The blood is purified by being brought, in successive instalments, into contact with pure air or pure water, the effect of which is to expel the carbonic acid produced by animal combustion, and to take up fresh supplies of oxygen. Whether the medium in which a mollusc lives be water or air, the effect of the respiratory action is practically the same.
Broadly speaking, Mollusca whose usual habitat is the water ‘breathe’ water, while those whose usual habitat is the land ‘breathe’ air. But this rule has its exceptions on both sides. The great majority of the fresh-water Mollusca which are not provided with an operculum (e.g. Limnaea, Physa, Planorbis), breathe air, in spite of living in the water. They make periodic visits to the surface, and take down a bubble of air, returning again for another when it is exhausted. On the other hand many marine Mollusca which live between tide-marks (e.g. Patella, Littorina, Purpura, many species of Cerithium, Planaxis, and Nerita) are left out of the water, through the bi-diurnal recess of the tide, for many hours together. Such species invariably retain several drops of water in their branchiae, and, aided by the moisture of the air, contrive to support life until the water returns to them. Some species of Littorina (e.g. our own L. rudis and many tropical species) live so near high-water mark that at neap-tides it must frequently happen that they are untouched by the sea for several weeks together, while they are frequently exposed to a burning sun, which beats upon the rocks to which they cling. In this case it appears that the respiratory organs will perform their functions[151] if they can manage to retain an extremely small amount of moisture.[265]
The important part which the respiratory organs play in the economy of the Mollusca may be judged from the fact that the primary subdivision of the Cephalopoda into Dibranchiata and Tetrabranchiata is based upon the number of branchiae they possess. Further, the three great divisions of the Gasteropoda have been named from the position or character of the breathing apparatus, viz. Prosobranchiata, Opisthobranchiata and Pulmonata, while the name Pelecypoda has hardly yet dispossessed Lamellibranchiata, the more familiar name of the bivalves.
Respiration may be conducted by means of—(a) Branchiae or Gills, (b) a Lung or Lung-cavity, (c) the outer skin.
In the Pelecypoda, Cephalopoda, Scaphopoda, and the great majority of the Gasteropoda, respiration is by means of branchiae, also known as ctenidia[266], when they represent the primitive Molluscan gill and are not ‘secondary’ branchiae (pp. 156, 159).
In all non-operculate land and fresh-water Mollusca, in the Auriculidae, and in one aberrant operculate (Amphibola), respiration is conducted by means of a lung-cavity, or rarely by a true lung, whence the name Pulmonata. The land operculates (Cyclophoridae, Cyclostomatidae, Aciculidae, and Helicinidae) also breathe air, but are not classified as Pulmonata, since other points in their organisation relate them more closely to the marine Prosobranchiata. Both methods of respiration are united in Ampullaria, which breathes indifferently air through a long siphon which it can elevate above the surface of the water, and water through a branchia (see p. 158). Siphonaria (Fig. 57) is also furnished with a lung-cavity as well as a branchia. Both these genera may be regarded as in process of change from an aqueous to a terrestrial life, and in Siphonaria the branchia is to a great extent atrophied, since the animal is out of the water, on the average, twenty-two hours out of the twenty-four. In the allied genus Gadinia, where there is no trace of a branchia, but[152] only a lung-cavity, and in Cerithidea obtusa, which has a pulmonary organisation exactly analogous to that of Cyclophorus,[267] this process may be regarded as practically completed.
Fig. 57.—A, Siphonaria gigas Sowb., Panama, the animal contracted in spirit: gr, siphonal groove on right side. B, Gadinia peruviana, Sowb., Chili, shell only: gr, mark of siphonal groove to right of head.
Respiration by means of the skin, without the development of any special organ, is the simplest method of breathing which occurs in the Mollusca. In certain cases, e.g. Elysia, Limapontia, and Cenia among the Nudibranchs, and the parasitic Entoconcha and Entocolax, none of which possess breathing organs of any kind, the whole outer surface of the body appears to perform respiratory functions. In others, the dorsal surface is covered with papillae of varied size and number, which communicate with the heart by an elaborate system of veins. This is the case with the greater number of the Aeolididae (Fig. 58, compare Fig. 5, C), but it is curious that when the animal is entirely deprived of these papillae, respiration appears to be carried on without interruption through the skin.
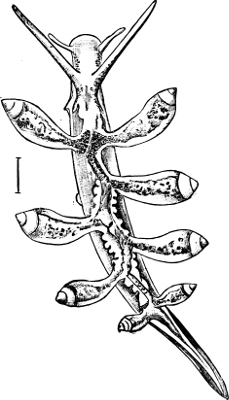
Fig. 58.—Aeolis despecta Johnst., British coasts. (After Alder and Hancock.)
In the development of a distinct breathing organ, it would seem as if progress had been made along two definite lines, each[153] resulting in the exposure of a larger length of veins, i.e. of a larger amount of blood, to the simultaneous operation of fresh air or fresh water. Either (a) the skin itself may have developed, at more or less regular intervals, elevations, or folds, which gradually took the form of papillae, or else (b) an inward folding, or ‘invagination,’ of the skin, or such a modification of the mantle-fold as is described below (p. 172) may have taken place, resulting in the formation of a cavity more or less surrounded by walls, within which the breathing organs were ultimately developed. Sometimes a combination of both processes seems to have occurred, and after a papilliform organ has been produced, an extension or prolongation of the skin has taken place, in order to afford a protection to it. Respiration by means of a lung-cavity is certainly subsequent, in point of time, to respiration by means of branchiae.
Fig. 59.—Chiton squamosus L., Bermuda: A, anus; Br, branchiae; M, mouth.
Fig. 60.—Fissurella virescens Sowb., Panama, showing position of the branchiae: Br, branchiae: E, E, eyes; F, foot; M, mantle; T, T, tentacles.
The branchiae seem to have been originally paired, and arranged symmetrically on opposite sides of the body. It is not easy to decide whether the multiple form of branchia which occurs in Chiton (Fig. 59), or the simple form as in Fissurella (Fig. 60), is the more primitive. Some authorities hold that the multiple branchia has gradually coalesced into the simple, others that the simple form has grown, by serial repetition, into the multiple. There appears to be no trace of any intermediate forms, and, as a matter of fact, the multiple branchia is found[154] only in the Amphineura, while one or rarely two (never more) pairs of branchiae, occur, with various important modifications, in the vast majority of the Mollusca.
Amphineura.—In Chiton the branchiae are external, forming a long row of short plumes, placed symmetrically along each side of the foot. The number of plumes, at the base of each of which lies an osphradial patch, varies from about 70 to as few as 6 or 7. When the plumes are few, they are confined to the posterior end, and thus approximate to the form and position of the branchiae in the other Amphineura. In Chaetoderma, the branchiae consist of two small feather-shaped bodies, placed symmetrically on either side of the anus, which opens into a sort of cloaca within which the branchiae are situated. In Neomenia the branchiae are still further degraded, consisting of a single bunch of filaments lying within the cloaca, while in Proneomenia there is no more than a few irregular folds on the cloaca-wall (Fig. 61).
Fig. 61.—Terminal portions of the Amphineura, illustrating the gradual degradation of the branchiae, and their grouping round the anus in that class. A, Chiton (Hemiarthrum) setulosus Carp., Torres Str.; B, Chiton (Leptochiton) benthus Hadd., Torres Str.; C, Chaetoderma; D, Neomenia; a, anus; br, br, branchiae; k, k, kidneys; p, pericardium. (A and B after Haddon, C and D after Hubrecht.)
In the Prosobranchiata, symmetrically paired branchiae occur only in the Fissurellidae, Haliotidae, and Pleurotomariidae, in the former of which two perfectly equal branchiae are situated on either side of the back of the neck. These three families taken together form the group known as Zygobranchiata.[268] In all other families the asymmetry of the body has probably caused one of the branchiae, the right (originally left), to become aborted, and consequently there is only one branchia, the left, in the vast majority of marine Prosobranchiata,[155] which have been accordingly grouped as Azygobranchiata. Even in Haliotis the right branchia is rather smaller than the left, while the great size of the attachment muscle causes the whole branchial cavity to become pushed over towards the left side. In those forms which in other respects most nearly approach the Zygobranchiata, namely, the Trochidae, Neritidae, and Turbinidae, the branchia has two rows of filaments, one on each side of the long axis, while in all other Prosobranchiata there is but one row (see Fig. 79, p. 169).
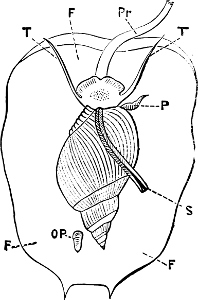
Fig. 62.—Bullia laevissima Gmel., showing branchial siphon S; F, F, F, foot; OP, operculum; P, penis; Pr, proboscis; T, T, tentacles. (After Quoy and Gaimard.)
In the great majority of marine Prosobranchiata the branchia is securely concealed within a chamber or pouch (the respiratory cavity), which is placed on the left dorsal side of the animal, generally near the back of the neck. For breathing purposes, water has to be conveyed into this chamber, and again expelled after it has passed over the branchia. In the majority of the vegetable-feeding molluscs (e.g. Littorina, Cerithium, Trochus) water is carried into the chamber by a simple prolongation of one of the lobes or lappets of the mantle, and makes its exit by the same way, the incoming and outgoing currents being separated by a valve-like fringe depending from the lobe. In the carnivorous molluscs, on the other hand, a regular tube, the branchial siphon, which is more or less closed, has been developed from a fold of the mantle surface, for the special purpose of conducting water to the branchia. After performing its purpose there, the spent water does not return through the siphon, but is conducted towards the anus by vibratile cilia situated on the branchiae themselves. In a large number of cases, this siphon is protected throughout its entire length by a special prolongation of the shell called the canal. Sometimes, as in Buccinum and Purpura, this canal is little more than a mere notch in the ‘mouth’ of the shell, but in[156] many of the Muricidae (e.g. M. haustellum, tenuispina, tribulus) the canal becomes several inches long, and is set with formidable spines (see Fig. 164, p. 256). In Dolium and Cassis the canal is very short, but the siphon is very long, and is reflected back over the shell.
The presence or absence of this siphonal notch or canal forms a fairly accurate indication of the carnivorous or vegetarian tendencies of most marine Prosobranchiata, which have been, on this basis, subdivided into Siphonostomata and Holostomata. But this classification is of no particular value, and is seriously weakened by the fact that Natica, which is markedly ‘holostomatous,’ is very carnivorous, while Cerithium, which has a distinct siphonal notch, is of vegetarian tendencies.
In the Zygobranchiata the water, after having aerated the blood in the branchiae, usually escapes by a special hole or holes in the shell, situated either at the apex (Fissurella) or along the side of the last whorl (Haliotis). In Pleurotomaria the slit answers a similar purpose, serving as a sluice for the ejection of the spent water, and thus preventing the inward current from becoming polluted before it reaches the branchiae (see Fig. 179, p. 266).
In Patella the breathing arrangements are very remarkable. In spite of their apparent external similarity, this genus possesses no such symmetrically paired plume-shaped branchiae as Fissurella, but we notice a circlet of gill-lamellae, which extends completely round the edge of the mantle. It has been shown by various authorities that these lamellae are in no sense morphologically related to the paired branchiae in other Mollusca, but only correspond to them functionally. The typical paired branchiae, as has been shown by Spengel, exist in Patella in a most rudimentary form, being reduced to a pair of minute yellow bodies on the right and left sides of the back of the ‘neck.’ A precisely similar abortion of the true branchiae, and special development of a new organ to perform their work, is shown in Phyllidia and Pleurophyllidia (see below under Opisthobranchiata). This circlet of functional gills in Patella has therefore little systematic value, being only developed in an unusual position, like the eyes on the mantle in certain Pelecypoda, to supply the place of the true organs which have fallen into disuse. Accordingly Cuvier’s class of Cyclobranchiata,[157] which included Patella and Chiton, has no value, and has indeed long been discarded. In Chiton the gills never extend completely round the animal, but are always more or less interrupted at the head and anus. They are the true gills, the plumes being serially repeated in the same way as the shell plates.
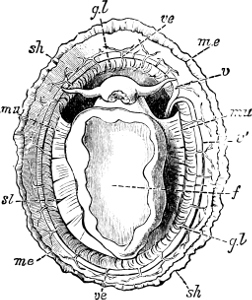
Fig. 63.—Patella vulgata L., seen from the ventral side: f, foot; g.l, circlet of gill lamellae; m.e, edge of the mantle; mu, attachment muscle; sl, slits in the same; sh, shell; v, vessel carrying aerated blood to the heart; v´, vessel carrying blood from the heart; ve, small accessory vessels.
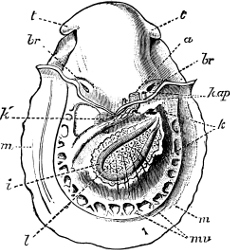
Fig. 64.—Patella vulgata L., seen from the dorsal side after the removal of the shell and the black pigment covering the integument; the anterior portion of the mantle is cut away or turned back: a, anus; br, br, remains of the true branchiae (ctenidia); i, intestine; k, k´, kidneys; k.ap, their apertures on each side of the anus; l, liver; m, m, mantle; mu, attachment muscles, severed in removal of shell; t, t, tentacles.
In the land Prosobranchiata (Cyclostomatidae, Cyclophoridae, Aciculidae, Helicinidae) which, having exchanged a marine for an aerial life, breathe air instead of water, the branchia has completely disappeared, and breathing is conducted, as in the Pulmonata, by a lung-cavity. In certain genera of land operculates, e.g. Pupina, Cataulus, Pterocyclus, a slight fissure or tube in the last whorl (see Fig. 180, p. 266) serves to introduce air into the shell, which is perhaps otherwise closed to air by the operculum. In Aulopoma, which has no tube, the operculum admits free circulation of air. In certain other Cyclostomatidae the apex is truncated, and air can enter there. De Folin closed with wax the aperture of Cycl. elegans, and found that on placing it in a pneumatic machine, the shell gave off air through its[158] whole surface. On the other hand, Cylindrella and Stenogyra decollata, on being submitted to the same test, showed that the truncated part alone was permeable by air.
Fischer and Bouvier have made some interesting observations on the breathing of a species of Ampullaria (insularum Orb.). The species has, in common with all Ampullaria, two siphons, but while the right siphon is but slightly developed, the left is very long, almost twice as long as the shell (see Fig. 65). The animal, when under the water, lengthens its siphon, brings the orifice to the surface, and by alternately raising and depressing its head produces in the pulmonary sac movements of ex- and inspiration; these are repeated about ten or fifteen times at regular intervals of from six to eight seconds, a method of respiration strongly resembling that of the Cetacea. At the same time, branchial respiration takes place. If powdered carmine is added to water, the particles are seen to enter the branchial cavity by the siphon and pass out by the short right siphon. Sometimes the animal remains under water for hours without rising to the surface to inspire air. In Valvata (Fig. 66) the branchia is very large, and projects like a leaf or fan above the shell on the left side; on the corresponding position on the right side is a long filiform appendage, which some have regarded as representing the other branchia.
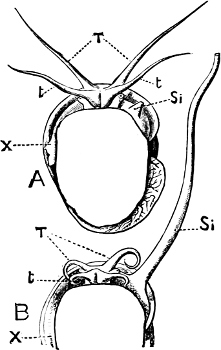
Fig. 65.—Ampullaria insularum Orb.: A, breathing water; B, breathing air; Si, siphon; T, upper; t, lower tentacles; X, pallial expansion, performing the part of excurrent siphon. (After Fischer and Bouvier, x ⅓.)
Opisthobranchiata.—A true branchia occurs only in the Tectibranchiata and the Ascoglossa. It lies on the right side, and is usually more or less external, being partly covered sometimes by the shell (as in Umbrella, Fig. 5), sometimes by a fold of the mantle. In the Pteropoda (which are probably derived from the Tectibranchiata), all the Thecosomata, with the exception of Cavolinia, have no specialised branchia, but probably respire through portions or the whole of the integument. In the Gymnosomata an accessory branchia has in many cases been developed at the posterior end of the body. Pneumodermon alone has[159] both lateral and posterior branchiae well developed, Clione and Halopsyche are destitute of either, while the four remaining families have one branchia, sometimes lateral, sometimes posterior.[269]
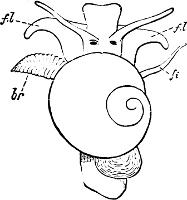
Fig. 66.—Valvata piscinalis Müll.: br, branchia; fi, filament; f.l, foot lobes. (After Boutan.)
Fig. 67.—Doris (Archidoris) tuberculata L., Britain: a, anus; br, branchiae, surrounding the anus; m, male organ; rh, rh, rhinophores. × ⅔.
Fig. 68.—Pleurophyllidia lineata Otto, Mediterranean: a, anus; br, secondary branchiae; m, mouth; s.o, sexual orifice.
Certain of the Nudibranchiata possess no special breathing organs, and probably respire through the skin (Elysia, Limapontia, Cenia, Phyllirrhoë). The majority, however, have developed secondary branchiae, in the form of prominent lobes or leaf-like processes (the cerata), which are carried upon the back, without any means of protection. These cerata are, as a rule, of extreme beauty and variety of form, consisting sometimes of long whip-like tentaculae, in other cases of arborescent plumes of fern-like leafage, in others of curious bead-like appendages of every imaginable shape and colour. In Doris they lie at the posterior end of the body, in a sort of rosette, which is generally capable of retraction into a chamber. In Phyllidia and Pleurophyllidia these secondary branchiae lie, as in Patella, on the lateral portions of the mantle.
[160]
The Scaphopoda in all probability possess neither true nor secondary branchiae.
Pulmonata.—When we use the term ‘lung,’ it must be remembered that this organ in the Mollusca does not correspond, morphologically, with the spongy, cellular lung of vertebrates; it simply performs the same functions. The ‘lung,’ in the Mollusca, is a pouch or cavity, lined with blood-vessels which are disposed over its vaulted surface in various patterns of network. The pulmonary sac or cavity is therefore a better name by which to denote this organ.

Fig. 69.—Geomalacus maculosus Allm., S. Ireland: P.O, pulmonary orifice.
It seems probable, as has been already shown (pp. 18–22), that all Pulmonata are ultimately derived from marine forms which breathed water by means of branchiae. Thus we find intermediate forms, such as Siphonaria, possessed of both a branchia and a pulmonary sac, the former being evanescent, while in Gadinia and Amphibola it has quite disappeared. In the vast majority of Pulmonata no trace of a branchia remains; its function is performed by a chamber, always situated at the right side of the animal, and generally more or less anterior, admitting air by a narrow aperture which is rhythmically opened and closed. In Arion and Geomalacus (Fig. 69) this aperture is in the front of the right side of the ‘shield,’ in Limax (Fig. 71) in the hinder part, in Testacella (Fig. 20) it is near the extremity of the tail, under the spire of the shell; in Janella it is on the middle of the right edge of the shield (Fig. 70). If a specimen of Helix aspersa, or better, of H. pomatia, is held up to the light, the beautiful arborescent vessels, with which the upper part of the pulmonary chamber is furnished, can be clearly seen by looking through the aperture as it dilates. It is only in the Auriculidae that an actual spongy mass of lung material appears to exist. When in motion, a Helix inspires air much more frequently than when at rest. Temperature, too, seems to affect the number of inspirations; it appears doubtful whether, during[161] hibernation, a snail breathes at all. In any case, the amount of air required to sustain life must be small.
Fig. 70.—Janella hirudo Fisch., N. Caledonia: G, generative orifice; P, pulmonary orifice; T, T, tentacles. (After Fischer.)
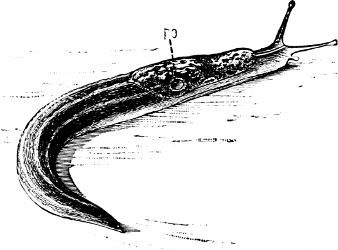
Fig. 71.—Limax maximus L.: PO, pulmonary orifice. × ⅔.
With regard to the respiration of fresh-water Pulmonata there appears to be some difference of opinion. It is held, on the one hand, that the Limnaeidae only respire air, making periodic visits to the surface to procure it, and that they perish, if prevented from doing so, by asphyxiation. If, we are told,[270] as a Limnaea is floating on the surface of the water in a glass jar, a morsel of common salt be dropped upon its outstretched foot, it will sink heavily to the bottom, emitting a stream of air from its pulmonary orifice. On recovering from the shock, it will anxiously endeavour to regain the surface, but will have some difficulty in doing so, owing to its now much greater specific gravity. When it succeeds, it creeps almost out of the water, and exposes its respiratory orifice freely to the air. If the experiment is repeated several times on the same individual, it becomes so much weakened that it has to be taken out of the water to save its life. Moquin-Tandon, on the other hand, is[162] strongly of opinion[271] that there is no absolute necessity for Limnaea to obtain air by rising to the surface, and that, if prevented from emerging, it can obtain air from the water. When covered in by a roof of ice, Limnaea has not been observed to suffer any inconvenience. Moquin-Tandon kept L. glabra and Planorbis rotundatus in good health under 20 mm. of water for eighteen and nineteen days, and relates a case in which Physa was kept alive under water for four days, and Planorbis for twelve. Young specimens, both of Limnaea and Planorbis, do not rise to the surface for a supply of air; they are hatched with the pulmonary cavity full of water.
It is probable, therefore, that Limnaeidae are capable, on occasion, of respiration through the skin. Some authorities are of opinion that certain long and narrow lamellae, situated within the pulmonary sac, are employed for the purpose of aqueous respiration. Ancylus, which never makes periodic excursions to the surface, perhaps respires by receiving into its pulmonary chamber the minute quantities of oxygen given off by the vegetation on which it feeds.
Limnaeidae taken from a great depth of water, e.g. from 130 fathoms in the lake of Geneva, have been examined by Forel.[272] The pulmonary sac is full of water, but there is no transformation of organs, no appearance of a branchia, to meet the changed circumstances of their environment. Doubtless a good deal of respiration is done by the skin; being soft and vascular, it respires the air dissolved in the water. Forel cites cases of Limnaea living at much shallower depths, which come to the surface once, and then remain below for months. The oxygen of this supply must soon have become exhausted, and the animals, discontinuing for a time the use of the pulmonary chamber, must have respired through the skin. Shallow-water Limnaea, according to the same authority, remain beneath the surface during cold weather; when warm weather returns they rise to the surface to take in a supply of air. Since the water at great depths is always very cold, there is no need for the Limnaea living there to rise to the surface at all.
It is a curious fact that Limnaea, which have been respiring by the skin for the whole winter, should suddenly, on the first warm days of summer, take to rising to the surface and breathing[163] air. But exactly the same phenomenon is shown in the case of Limnaea from great depths. Placed in an aquarium, they immediately begin rising to the surface and inspiring air; in other words, they experience instantaneously a complete transformation of their respiratory system.
In Onchidium, a land pulmonate which has retrogressed to an amphibious or quasi-marine mode of life, there is no organ which represents the pulmonary or branchial cavity, the so-called lung being only a cavity of the kidney. Respiration is, however, conducted by the skin as well, and by the dorsal papillae.[273]
Land Mollusca can sustain, for a considerable time, complete deprivation of atmospheric air. Helices placed in an exhausted receiver show no signs of being inconvenienced for about 20 hours, and are able to survive for about two or three days. If detained under water, they are very active for about 6 hours, then become motionless, the body swells, owing to the water absorbed, and death ensues in about 36 hours. Immersion for only 24 hours is generally followed by recovery. In the latter case, the cause of death is not so much deprivation of air as compulsory absorption of water by the skin. The amount of water thus taken up is surprising. Spallanzani found that a Helix which weighed 18 grammes increased in weight by 13½ grammes after a prolonged immersion. Even slugs enclosed in moist paper gained more than 2 grammes in the course of half an hour. Experiment has shown that the amount of carbonic acid gas produced by respiration stands in direct relation to the amount of food consumed. Four pairs of snails were taken which had recently awakened from their winter sleep and had eaten heartily, and an equal number, under the same circumstances, which had been prevented from eating. It was found that the first four pairs produced, in consuming a given amount of oxygen, 11, 9, 10, and 13 parts respectively of carbonic acid, while the second set produced, in consuming the same amount of oxygen, only 4, 8, 7, and 9 parts of carbonic acid.[274] Hibernating Helices, if weighed in December and again in April, will be found to have lost weight, due to the expiration of carbonic acid. Owing to the difficulty of experiment, opinions vary as to the absolute temperature of snails. It appears to be established that several snails, if placed together in a tube, raise the[164] temperature one or two degrees C., but as a rule, the temperature of a solitary Helix differs very slightly from that of the surrounding air. Increased activity, whether in respiration or feeding, is found to raise the temperature.
Fig. 72.—Cardium edule L.: A, anal; B, branchial siphon; F, foot. (After Möbius.)
W. H. Dall, writing of the branchia in Pelecypoda, remarks[275] that there can be no doubt that its original form was a simple pinched-up lamella or fold of the skin or mantle. This, elongated, becomes a filament. Filaments united by suitable tissue, trussed, propped, and stayed by a chitinous skeleton, result in the forms, wonderful in number and complexity, which puzzle the student to describe, much more to classify.
Fig. 73.—Scrobicularia piperata Gmel., in its natural position in the sand: A, efferent or anal siphon; B, afferent or branchial siphon. (After Möbius.)
In Pelecypoda the branchiae are placed on each side of the body, between the mantle and the visceral mass. They lie in a chamber known as the branchial cavity. Leading into this cavity, and behind it, are, as a rule, two tubes or siphons, one of which conducts water to the branchiae, while the other carries it away after it has passed over them. The lower is known as the branchial or afferent siphon, the upper as the anal or efferent siphon (see Figs. 72 and 73). The action of these siphons can readily be observed by placing a little carmine in[165] water, near to the siphonal apertures of an Anodonta or Unio. In many cases (e.g. Psammobia, Tellina, Mya, genera which burrow deeply in sand) both the siphons are exceedingly long, sometimes considerably longer than the whole shell. In some cases the two tubes are free throughout their entire length, in others they become fused together before their entrance within the shell (Fig. 74). In other genera, which do not burrow (e.g. Ostrea, Pecten, Arca, Mytilus), the siphons are rudimentary or altogether absent (Fig. 75).
Fig. 74.—Solecurtus strigillatus L., Naples: s.af, afferent siphon; s.ef, efferent siphon, the two uniting in SS externally to the shell, × ½.
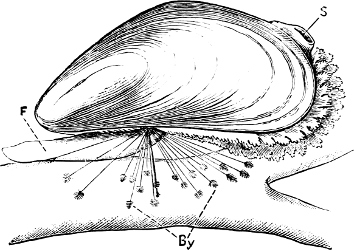
Fig. 75.—Mytilus edulis L., attached by its byssus (By) to a piece of wood: F, foot; S, anal siphon, the branchial siphon being below it and not closed. (After Möbius.)
The number and arrangement of the branchiae varies considerably. It appears probable that the different degrees of complication of the gill indicate degrees of specialisation in the different groups of Pelecypoda, in other words, assuming that a simpler form of gill precedes, in point of development, a more complicated form, the nature of the gill may be taken as indicating different degrees of removal from the primitive form of bivalve.
[166]
1. The simplest form of gill (Nucula, Leda, Solenomya, etc.) is that which consists (Fig. 76, A, compare Fig. 100, p. 201) of two rows of very short, broad, not reflected filaments, the rows being placed in such a way that they incline at right angles to one another from a common longitudinal axis. The filaments are not connected with one another, nor are the two leaves of each gill united at any point. (Protobranchiata.)
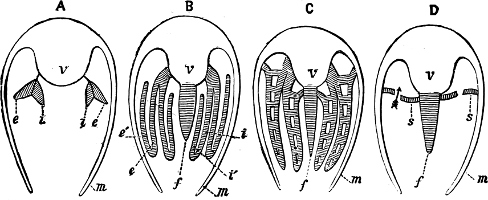
Fig. 76.—Morphology of the branchiae of Pelecypoda, seen diagrammatically in section: A, Protobranchiata; B, Filibranchiata; C, Eulamellibranchiata; D, Septibranchiata; e, e, external row of filaments; i, i, internal row of filaments; e´, external row or plate folded back; i´, internal row folded back; f, foot; m, mantle; s, septum; v, visceral mass. (From A. Lang.)
Fig. 77.—Four gill filaments of Mytilus, highly magnified; cj, ciliary junctions; f, filament. (After Peck.)
2. In the Anomiidae, Arcadae, Trigoniidae, and Mytilidae each gill consists of two plates or rows of much longer filaments, which consequently occupy a much larger space in the mantle cavity (Fig. 76, B). Unable to extend beyond the limits of the mantle, filaments are reflected or doubled back upon one another, those of the external plate being reflected towards the outside, those of the internal plate towards the inside. Each separate filament is not connected with the filament next adjacent, except by surface cilia situated on small projections on the sides of the filaments, and interlocking with the cilia of the adjacent filament. The two superposed plates or leaves of the gill may or may not be united by cords running between the two parts of a filament. (Filibranchiata.)
3. In the Pectinidae, Aviculidae, and Ostreidae a further development takes place. The filaments of each gill are reflected in the same way as in the Filibranchiata, but the part thus reflected may become completely[167] united or ‘concresce’ with the mantle on the exterior and with the base of the foot on the interior side. The leaves of each gill plate, which have thus become doubled (the gills being apparently two instead of one on each side), are folded or crumpled, and the filaments are modified at the re-entrant angles of the fold. (Pseudolamellibranchiata.)
4. In all the remaining Pelecypoda, except class 5, in other words, in the very large majority of families, the filaments are either reflected, as in (3), or simple; but the process of concrescence is so far advanced that the adjacent filaments are always intimately connected with one another in such a way as to admit the passage of the blood; and the leaves of each gill-plate (Fig. 76, C) are united by cross channels in a similar way. (Eulamellibranchiata.)
5. In certain of the Anatinacea alone (Cuspidaria, Lyonsiella, Poromya, Silenia) the gills are transformed into a more or less muscular partition, extending from one adductor muscle to the other (Fig. 76, D), and separating off the pallial chamber into two distinct divisions, which communicate by means of narrow slits in the partition. (Septibranchiata.)
Fig. 78.—Transverse section of portion of an outer gill plate of Anodonta, highly magnified: il, inner lamella; il´, outer lamella; ilj, interlamellar junctions; v, large vertical vessels. (After Peck.)
Thus the process of gill development in the Pelecypoda appears to lead up from a simple to a very complex type. In its original form, at all events in the most primitive form known to us, the gill is a series of short filaments, quite independent of one another, strung in two rows; then the filaments become longer and double back, while at the same time they begin to show signs of adhesion, as yet only superficial, to one another. In a further stage, the reflected portions become fused to the adjacent surfaces of the foot and mantle, while the interlamellar junctions serve to lock the two gill-plates together; finally, the mere ciliary junction of adjacent filaments is exchanged for intimate vascular connection, while the gill-plates as a whole become closely fused together in a similar manner.
This theory of origin is strengthened by closer observation[168] of the phenomena of a single group. Taking the Septibranchiata as an instance, we find that in Lyonsiella the branchiae unite with the mantle in such a way as to form two large pallial chambers, the structure of the branchiae being preserved, and their lamellae covering the partition. A further stage is observed in Poromya. There, a similar partition exists, but it has become muscular, preserving, however, on each side two groups of branchial lamellae, separated one from the other by a series of slits, which form a communication between the two pallial chambers. A further stage still is seen in Silenia. There the same muscular partition exists, but the branchial lamellae on either side have disappeared, the slits between the two chambers, which occur in Poromya, still persisting, but separated into three groups. Cuspidaria represents the last stage in the development. In the ventral chamber there appears nothing at all corresponding to a branchia; the surface of the partition appears perfectly uniform, but on careful examination three little separate orifices, remains of the three groups of orifices in Silenia, are observed.[276]
Relation between Branchiae and Heart.—The object of the branchiae being, as has been already stated, to aerate the blood on its way to the heart, we find that the heart and the branchiae stand in very important structural relations to one another. When the branchiae are in pairs, we find that the auricles of the heart are also paired, the auricle on the right and left sides being supplied by the right and left branchiae respectively. This is the case with the Dibranchiate Cephalopods (Argonauta, Octopus, Loligo, etc.), the Zygobranchiate Prosobranchs (Fissurella, Haliotis), and all Pelecypoda. In the Amphineura (Chiton, etc.) there are two auricles corresponding to the two sets of multiple branchiae. In the case of the Tetrabranchiate Cephalopods (Nautilus) there are four auricles corresponding to each of the four branchiae. Compare Fig. 79, A, B, C, D, E.
On the other hand, when the branchia is single, or when both branchiae are on the same side, and one is aborted and functionless, the auricle is single too, and on the same side as the branchia. This is the case with the Tectibranchiate Opisthobranchs (Philine, Scaphander, etc.), all the Pectinibranchiate Prosobranchs (Rachiglossa, Taenioglossa, and Ptenoglossa), and[169] the other Azygobranchiate Prosobranchs (Trochidae, Neritidae, etc.). In the last case the right auricle exists, as well as the left, but is simply a closed sac, the coalescing of the two gills on the left side having thrown all the work upon the left auricle. Compare Fig. 79, F, G, H.
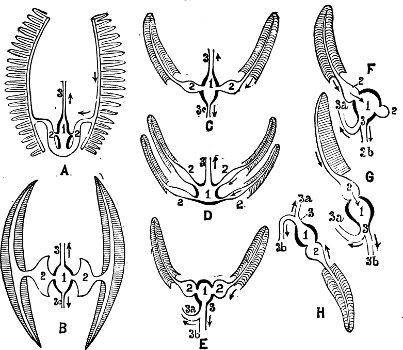
Fig. 79.—Diagram illustrating the relations between branchiae, heart, and aorta in the Mollusca: A, In Chiton; B, Pelecypoda; C, Dibranchiate Cephalopoda; D, Tetrabranchiate Cephalopoda; E, Prosobranchiata Zygobranchiata; F, Prosobranchiata Azygobranchiata; G, Prosobranchiata Monotocardia; H, Opisthobranchiata Tectibranchiata: 1, Ventricle; 2, Auricle; 3, Aorta; 3a, Cephalic aorta; 3b, Visceral aorta; 3c, Posterior aorta. (From A. Lang.)
All Mollusca, without exception, possess a circulatory system of more or less complexity. The centre of the system is the heart, which receives the aerated blood from the breathing organs, and propels it to every part of the body. In the Scaphopoda alone there appears to be no distinct heart.
The heart may consist simply of a single auricle and ventricle, and an aorta opening out of the ventricle. From the aorta the blood is conveyed to the various parts of the body by arteries. Veins convey the blood back to the breathing organs, after passing over which it returns by the branchial or pulmonary vein to the heart, thus completing the circuit.
[170]
As regards position, the heart is situated within the pericardium, a separate chamber which in the Pelecypoda, Cephalopoda, and the bilaterally symmetrical Gasteropoda lies on the median line, while in the asymmetrical Gasteropoda it is on one or other of the sides of the body, usually the right. The veins connected with the branchiae, and consequently the auricle into which they open, are situated behind the ventricle in the Opisthobranchiata (whence their name), while in the Prosobranchiata they are situated in front of the ventricle.
The number of auricles corresponds to the number of branchiae. Thus there is only one auricle in the great majority of Prosobranchiata (which are accordingly classified as Monotocardia), and also in the Opisthobranchiata, while the Pulmonata have a single auricle corresponding to the pulmonary chamber. There are two auricles in the Amphineura, in a small group of Gasteropoda, hence known as Diotocardia, in all Pelecypoda, and in the Dibranchiate Cephalopoda. In the Tetrabranchiate Cephalopoda alone there are four auricles corresponding to the four branchiae.
A single aorta occurs only in the Amphineura and in the Tetrabranchiate Cephalopoda. In all the other groups there are two aortae, leading out of the anterior and posterior ends of the ventricle in Pelecypoda and Dibranchiate Cephalopoda, while a single aorta leads out of the posterior end alone, and subsequently bifurcates, in most of the Gasteropoda. One aorta, the cephalic, supplies the front part of the body, the oesophagus, stomach, mantle, etc.; the other, the visceral aorta, supplies the posterior part, the liver and sexual organs.
The general circulatory system in the Mollusca has not yet been thoroughly investigated. As a general rule, the blood driven from the ventricle through the aorta into the arteries, passes, on reaching the alimentary canal and other adjacent organs, into a number of irregular spaces called lacunae. These in their turn branch into sinuses, or narrow tubes covered with muscular tissue, which penetrate the body in every direction. In the Dibranchiate Cephalopoda true capillaries are said to occur, which in some cases form a direct communication between the arteries and veins. According to some authorities[277] capillaries and veins exist in certain Pelecypoda in connexion with the intestinal lacunae, but this again is regarded by others as not established. A similar[171] difference of opinion occurs with regard to the precise function of the foot-pore which occurs in many Mollusca, some holding that it serves as a means for the introduction of water into the blood-vascular system, while others regard it as a form of secretion gland, the original purpose of which has perhaps become lost.
Blood.—As a rule, the blood of the Mollusca—i.e. not the corpuscles but the liquor sanguinis—is colourless, or slightly tinged with blue on exposure to the air. This is due to the presence of a pigment termed haemocyanin, in which are found traces of copper and iron, the former predominating. Haemoglobin, the colouring matter of the blood in Vertebrates, is, according to Lankester,[278] of very restricted occurrence. It is found—(1) in special corpuscles in the blood of Solen legumen (and Arca Noae); (2) in the general blood system of Planorbis; (3) in the muscles of the pharynx and jaws of certain Gasteropoda, e.g. Limnaea, Paludina, Littorina, Chiton, Aplysia. This distribution of haemoglobin is explained by Lankester in reference to its chemical activity; whenever increased facilities for oxidisation are required, then it may be present to do the work. The Mollusca, being as a rule otiose, do not possess it generally diffused in the blood, as do the Vertebrata. The actively burrowing Solen possesses it, and perhaps its presence in Planorbis is to be explained from its respiring the air of stagnant marshes. Its occurrence in the pharyngeal muscles and jaws of other genera may be due to the constant state of activity in which these organs are kept.[279]
According to Tenison-Woods[280] a species of Arca (trapezia Desh.) and two species of Solen, all Australian, have red blood. It is suggested that in these cases the habits of the animal (the Solen burrowing deeply in sand, the Arca in mud) require some highly oxidising element, surrounded as the creature is by ooze. In Arca pexata (N. America) the blood is red, the animal being familiarly known as the ‘bloody clam.’ Burrowing species, however, are not all distinguished by this peculiarity. Tenison-Woods finds red fluids in the buccal mass of many Gasteropoda, e.g. in species of Patella, Acmaea, Littorina, Trochus, Turbo, giving the parts the appearance of raw meat.
[172]
On the dorsal side of the typical molluscan body, between the visceral sac and the shell, lies a duplicature of the integument, generally known as the mantle. The depending sides of the mantle, which are usually somewhat thickened, enclose between themselves and the body mass a chamber of varying size and shape, called the mantle cavity, which communicates freely with the external air or water, and encloses and furnishes a protection for the organ or organs of respiration. On its upper or dorsal surface the mantle is closely applied to the shell throughout its whole extent, the cells with which it is furnished secreting the materials from which the shell is formed (see p. 255). The whole mantle is capable, to some degree, of secreting shelly matter, but the most active agent in its production is the mantle edge or margin.
In the Prosobranchiata the mantle cavity, for reasons which have already been explained, is found on the left side of the animal, its front portion being in many cases produced into a tubular siphon. Within the mantle cavity are found, besides the branchia, the anus, the apertures of the kidneys, and the osphradium. In the pulmonata the mantle fold encloses a so-called lung-cavity. The front edge of the mantle coalesces with the integument of the neck in such a way as to enclose the cavity very completely, the only communication with the outer air being by means of the contractile breathing or pulmonary aperture on the right side. In the Tectibranchiate Opisthobranchs the mantle fold is inconsiderable, and is usually not of sufficient extent to cover the branchia, while in the Nudibranchs, which have no true branchiae, it disappears altogether.
In the Pelecypoda the mantle cavity is equally developed on each side, enclosing the two sets of branchiae. The mantle may thus be regarded as consisting of two equal portions, which form a sort of lining to the two valves. The lower or ventral portion of the mantle edges may be simple, or provided with ocelli (Pecten, Arca), tentacles, cilia (Lima, Lepton), or doubled folds. The two portions of the mantle touch one another along the whole line of the edge of the two valves, and, although thus in contact, may remain completely separate from one another, or[173] else become permanently united at one or more points. This fusion of the mantle edges corresponds to important changes in the organisation of the animal as a whole. The anal and branchial siphons are no more than prolongations of the mantle edges on the posterior side into a tubular form. These ‘siphons’ exhibit the siphonal form more distinctly according as the adjacent portions of the mantle become more definitely fused together.
Fig. 80.—Diagram illustrating the various stages in the closing of the mantle in Pelecypoda: A, mantle completely open; B, rudiments of siphons, mantle still completely open; C, mantle closed at one point; D, mantle closed at two points, with complete formation of siphonal apertures; E, development of siphons, ventral closure more extended; F, mantle closed at three points, with fourth orifice: f, foot; s.a, s.b, anal and branchial siphons; 1, 2, 3, first, second, and third points of closure of mantle. (After A. Lang.)
This progressive fusion of the mantle edges may be taken as indicating definite stages in the development of the Pelecypoda. A perfectly free mantle edge, joined at no point with the edge of the adjacent mantle, occurs in Nucula, Arca, Anomia, and Trigonia (see Fig. 80, A, B). Here there is nothing in the nature of a siphon, either anal or branchial; in other words, no contrivance exists to prevent the spent water which has passed over the branchiae from becoming mixed with the fresh water which is to reach them. When the mantle edges are fused at one point only, this is invariably on the middle part of the posterior side, thus separating off an anal opening which may become prolonged into a tube-like form. At the same time the adjacent underlying portions of the mantle edges draw together, without actually coalescing, to form an opening for the incurrent stream of water, the rudiments of the ‘branchial siphon’ (Fig. 80, C). This is the case with most Mytilidae (see Fig. 75) with Cardita, Astarte, and Pisidium. In the next stage the branchial opening is separated off by the concrescence[174] of the mantle edges beneath it, and we have the mantle united in two places, thus forming three openings, the ventral of which is the opening for the protrusion of the foot (Fig. 80, D). This is the case in Yoldia, Leda, the majority of the Eulamellibranchiata (e.g. Lucina, Cyrena, Donax, Psammobia, Tellina, Venus, Cardium, Mactra), and all Septibranchiata. In Chama and Tridacna the fused portions of the mantle become more extended, and in Pholas, Xylophaga, Teredo, Pandora, and Lyonsia this concrescence takes place over the greater length of the whole mantle edge, so that the mantle may be regarded as closed, with the exception of the three apertures for the foot and the two siphons (Fig. 80, E).
In certain genera there occurs, besides these three apertures, a fourth, in the line of junction between the pedal and branchial orifices. It appears probable that this fourth orifice (which has been regarded by some as an inlet for water when the siphons are retracted), stands in relation to the byssal apparatus (Fig. 80, F). In Lyonsia, for instance, a thick byssus protrudes through the orifice, which is large and open. In Solen, Lutraria, Glycimeris, Cochlodesma, Thracia, Aspergillum, and a few more genera, which have no byssus, the orifice is very small and narrow. It is possible that in these latter cases, the byssal apparatus having become atrophied, the orifice has been correspondingly reduced in size.[281]
Mantle Reflected over the Shell.—It is sometimes the case that the mantle edges tend to double back over the external surface of the shell, and to enclose it to a greater or less extent. When this process is carried to an extreme, the edges of the reflected mantle unite, and the shell becomes completely internal. We see an incipient stage of this process in Cypraea and Marginella, where the bright polish on the surface of the shell is due to the protection afforded by the lobes of the mantle. A considerable portion of the shell of Scutus is concealed in a similar way, while in Cryptochiton, Lamellaria, and Aplysia the shell is more or less completely enclosed. Among Pulmonata, it is possible that in forms like Vitrina, Parmacella, Limax, and Arion, we have successive stages in a process which starts with a shell completely external, as in Helix, and ends, not merely by enveloping the shell in the mantle, but by effecting its disappearance[175] altogether. In Vitrina and some allied genera we have a type in which the mantle lobes are partly reflected over the shell, which at the same time exhibits rather less of a spiral form than in Helix. In the stage represented by Parmacella, the mantle edges have coalesced over the whole of the shell, except for a small aperture immediately over the spire; the nucleus alone of the shell is spiral, the rest considerably flattened. In Limax the shell has become completely internal, and is simply a flat and very thin plate, the spiral form being entirely lost, and the nucleus represented by a simple thickening at one end of the plate. In Arion, the final stage, we find that the shell, being no longer needed as a protection to the vital organs, has either become resolved into a number of independent granules, or else has entirely disappeared.
Some indications of a similar series of changes occur in the Pelecypoda. The mantle edge of Lepton is prolonged beyond the area of the valves, terminating in some cases in a number of filaments. In Galeomma and Scintilla the valves are partially concealed by the reflected mantle lobes, and in a remarkable form recently discovered by Dall[282] (Chlamydoconcha) the shell is completely imbedded in the mantle, which is perforated at the anterior end by an orifice for the mouth, and at the posterior end by a similar orifice for the anus. In all these cases, except Lepton, it is interesting to notice that the hinge teeth have completely disappeared, the additional closing power gained by the external mantle rendering the work done by a hinge unnecessary. It is quite possible, on the analogy of the Gasteropoda mentioned above, and also, it may be added, of the Cephalopoda and other groups, that we have here indicated the eventual occurrence of a type of Pelecypoda altogether deprived of valves, a greatly thickened mantle performing the part of a shell.[283]
The following works will be found useful for further study of this portion of the subject:—
F. Bernard, Recherches sur les organes palléaux des Gastéropodes prosobranches: Ann. Sc. Nat. Zool. (7) ix. (1890), pp. 89–404.
[176]
G. Cuvier, Le Régne animal (ed. V. Masson); Mollusca, Text and Atlas.
C. Grobben, Beiträge zur Kenntniss des Baues von Cuspidaria (Neaera) cuspidata Olivi, nebst Betrachtungen über das System der Lamellibranchiaten: Arb. Zool. Inst. Wien, x. (1893), pp. 101–146.
E. Ray Lankester, Encyclopaedia Britannica, 9th ed., vol. xvi. (1883), Art. ‘Mollusca.’
A. Ménégaux, Recherches sur la circulation des Lamellibranches marins: Besançon, 1890.
K. Mitsukuri, On the structure and significance of some aberrant forms of Lamellibranchiate gills: Q. Journ. Micr. Sc., N.S. xxi. (1881), pp. 595–608.
H. L. Osborn, On the gill in some forms of Prosobranchiate Mollusca: Stud. Biol. Lab. Johns Hopk. Univ. iii. (1884), pp. 37–48.
R. Holman Peck, The structure of the Lamellibranchiate gill: Q. Journ. Micr. Sc., N.S. xvii. (1877), pp. 43–66.
P. Pelseneer, Contributions à l’étude des lamellibranches: Arch. Biol. xi. (1891), pp. 147–312.
[177]
Tactile organs, although occurring in some of the Mollusca, do not appear to attain special or marked development, except in a few cases. The whole surface of the skin, and particularly of the foot, is very sensitive to the slightest impression. Nearly all Gasteropoda are furnished with at least two cephalic tentacles, projecting like horns from each side of the fore part of the head. At or near the base of these are generally situated the eyes. In the Helicidae the eyes are situated, not at the base, but at the apex of the tentacles, and in that case—except in Vertigo—a second pair of shorter tentacles appears beneath the longer pair. It frequently happens that several senses are centred in a single organ; thus the upper tentacles of snails not only carry the eyes and serve to a certain extent as tactile organs, but they also carry the organs of smell.
The edges of the mantle, which are sometimes specialised into lobes, appear to be keenly sensitive to touch in all Gasteropoda.
In Cypraea (Fig. 81) these lobes, or tentaculae, are a prominent feature of the animal, and also in certain genera of the Trochidae (Fig. 82). In most of the carnivorous land Pulmonata—e.g. Testacella, Rhytida, Ennea—there are developed, under the lower pair of tentacles, and close to the mouth, large labial palps or feelers. These are connected with the cerebral ganglion by a very large nerve, and may therefore be supposed to be of extreme sensitiveness. In some of the large carnivorous forms[178] (Glandina, Aerope, compare Fig. 21, p. 54) these palpae are of great size, and curl upwards like an enormous pair of moustaches. When a Glandina seizes its prey, the palpae (see Fig. 83) appear to enfold it and draw it in towards the mouth.
Fig. 81.—Cypraea moneta L., showing tentaculae at edge of mantle, which partly envelops the shell: Si, siphon; M, M, mantle; F, foot; T´, tentaculae at edge of mantle. (After Quoy and Gaimard.) × 3/2.
Fig. 82.—Monodonta canalifera Lam., New Ireland, showing mantle lobes. (After Quoy and Gaimard.)
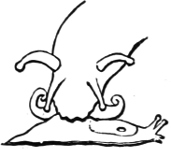
Fig. 83.—Glandina seizing its prey, with buccal papillae turned back. (Strebel.)
It is in the Opisthobranchiata that the organs of touch attain their maximum development. Many of this group are shell-less or possess a small internal shell, and accordingly, in the absence of this special form of defence, a multiplied sense of touch is probably of great service. Thus we find, besides the ordinary cephalic tentacles, clusters or crowns of the same above the head of many Nudibranchiata, with lobe-like prolongations of the integument, and tentacular processes in the neighbourhood of, or surrounding the branchiae (see Figs. 58 and Fig. 84, or even projecting from the whole upper surface of the body (Fig. 5, C).
In the Pelecypoda, the chief organs of touch are the foot, which is always remarkably sensitive, especially towards its point, the labial palps on each side of the mouth, and the siphons. In certain cases the mantle border is prolonged into a series of threads or filaments. These are particularly noticeable in Pecten, Lepton, and Lima (Fig. 85), the mantle lobes of the common L. hians of our own coasts being very numerous, and of a bright orange colour. In many genera—e.g. Unio, Mactra—this sensibility to touch appears to be shared by the whole mantle border, although it is not furnished with any special fringing. The ‘arms’ of the Cephalopoda[179] appear to be keenly sensitive to touch, and this is particularly the case with the front or tentacular pair of arms, which seem to be employed in an especial degree for exploration and investigation of strange objects.
Fig. 84.—Idalia Leachii A. and H., British seas; br, branchiae. (After Alder and Hancock.)
Fig. 85.—Lima squamosa Lam., Naples, showing tentacular lobes of mantle (t, t); a, anus; ad.m, adductor muscle; br, br, branchiae; f, foot; sh, shell.
Taste.—The sense of taste is no doubt present, to a greater or less extent, in all the head-bearing Mollusca. In many of these a special nerve or nerves has been discovered in the pharynx, connecting with the cerebral ganglion; this no doubt indicates the seat of the faculty of taste. The Mollusca vary greatly in their likings for different kinds of food. Some seem to prefer decaying and highly odoriferous animal matter (Buccinum, Nassa), others apparently confine themselves to fresh meat (Purpura, Natica, Testacella), others again, although naturally vegetarian, will not refuse flesh on occasion (Limax, Helix).
Mr. W. A. Gain[284] has made some interesting experiments on the taste of British land Mollusca, as evidenced by the acceptance or rejection of various kinds of food. He kept twelve species of Arion and Limax, and eight species of Helix in captivity for many months, and tried them with no less than 197 different kinds of food, cannibalism included.[180] Some curious points came out in his table of results. Amalia gagates appears to be surprisingly omnivorous, for out of 197 kinds of food it ate all but 25; Arion ater came next, eating all but 40. Limax arborum, on the other hand, was dainty to a fault, eating only seven kinds of food, and actually refusing Swedes, which every other species took with some avidity. Certain food was rejected by all alike, e.g. London Pride, Dog Rose, Beech and Chestnut leaves, Spruce Fir, Common Rush, Liverwort, and Lichens; while all, or nearly all, ate greedily of Potatoes, Turnips, Swedes, Lettuces, Leeks, Strawberries, Boletus edulis, and common grasses. Few of our common weeds or hedgerow flowers were altogether rejected. Arion and Limax were decidedly less particular in their food than Helix, nearly all of them eating earthworms and puff-balls, which no Helix would touch. Arion ater and Limax maximus ate the slime off one another, and portions of skin. Cyclostoma elegans and Hyalinia nitida preferred moist dead leaves to anything else.
Position of Eyes.—In the majority of the head-bearing Mollusca the eyes are two in number, and are placed on, or in the immediate neighbourhood of the head. Sometimes they are carried on projecting tentacles or ‘ommatophores,’ which are either simple (as in Prosobranchiata) or capable of retraction like the fingers of a glove (Helix, etc.). Sometimes, as in a large number of the marine Gasteropoda, the eyes are at the outer base of the cephalic tentacles, or are mounted on the tentacles themselves, but never at the tip (compare Fig. 60, p. 153 and[181] Fig. 98, p. 199). In other cases they are placed somewhat farther back, at the sides of the neck. The Pulmonata are usually subdivided into two great groups, Stylommatophora and Basommatophora (Fig. 86), according as the eyes are carried on the tip of the large tentacles (Helix, and all non-operculate land shells), or placed at the inner side of their base (Limnaea, Physa, etc.). In land and fresh-water operculates, the eyes are situated at the outer base of the tentacles.
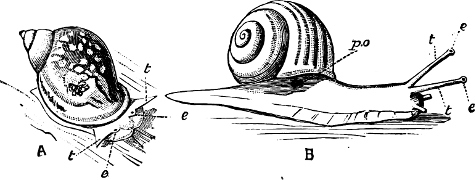
Fig. 86.—A, Limnaea peregra Müll.; e, e, eyes; t, t, tentacles; B, Helix nemoralis Müll.; e, e, eyes; t, t, tentacles; p.o, pulmonary orifice.
In the Helicidae, careful observation will show that the eyes are not placed exactly in the centre of the end of the tentacle, but on its upper side, inclining slightly outwards. The eye is probably pushed on one side, as it were, by the development of the neighbouring olfactory bulb. The sense of smell being far more important to these animals than the sense of sight, the former sense develops at the expense of the latter.
Organisation of the Molluscan Eye.—The eye in Mollusca exhibits almost every imaginable form, from the extremely simple to the elaborately complex. It may be, as in certain bivalves, no more than a pigmented spot on the mantle, or it may consist, as in some of the Cephalopoda, of a cornea, a sclerotic, a choroid, an iris, a lens, an aqueous and vitreous humour, a retina, and an optic nerve, or of some of these parts only.
In most land and fresh-water Mollusca the eye may be regarded, roughly speaking, as a ball connected by an exceedingly fine thread (the optic nerve) with a nerve centre (the cerebral ganglion). In Paludina this ball is elliptic, in Planorbis and Neritina it is drawn out at the back into a conical or pear shape. In Helix (Fig. 87) there is a structureless membrane, surrounding the whole eye, a lens, and a retina, the latter consisting of a nervous layer, a cellular layer, and a layer of rods containing pigment, this innermost layer (that nearest the lens) being of the thickness of half the whole retina.
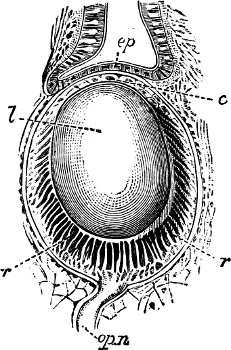
Fig. 87.—Eye of Helix pomatia L., retracted within the tentacle; c, cornea; ep, epithelial layer; l, lens; op.n, optic nerve; r, retina. (After Simroth.)
Comparing the eyes of different Gasteropoda together, we[182] find that they represent stages in a general course of development. Thus in Patella the eye is scarcely more than an invagination or depression in the integument, which is lined with pigmented and retinal cells. The next upward stage occurs in Trochus, where the depression becomes deeper and bladder-shaped, and is filled with a gelatinous or crystalline mass, but still is open at the top, and therefore permits the eye to be bathed in water. Then, as in Turbo, the bladder becomes closed by a thin epithelial layer, which finally, as in some Murex, becomes much thicker, while the ‘eyeball’ encloses a lens (Fig. 88), which probably corresponds with the ‘vitreous humour’ of other types.
Fig. 88.—Eyes of Gasteropoda, showing arrest of development at successive stages: A, Patella; B, Trochus; C, Turbo; D, Murex; ep, epidermis; l, lens; op.n, optic nerve; r, retina; v.h, vitreous humour. (After Hilger.)
In Nautilus the eye is of a very simple type. It consists of a cup-shaped depression, with a small opening which is not quite closed by the integument. The retina consists of cells[183] which line the interior of the depression, and which communicate directly with the branches of the optic nerve, there being no iris or lens. This type of eye, it will be observed, corresponds exactly with that which occurs in Patella. It appears also to correspond to a stage in the development of eyes in the Dibranchiata (e.g. Octopus, Sepia, Loligo). Lankester has shown[285] that in Loligo the eye first appears as a ridge, enclosing an oval area in the integument. By degrees the walls of this area close in, and eventually join, enclosing the retinal cells within the chamber in which the lens is afterwards developed (Fig. 89). It thus appears that in some cases the development of the eye is arrested at a point which in other cases only forms a temporary stage towards a higher type of organisation.
Fig. 89.—Three stages in the development of the eye of Loligo; r, r, ridge, enclosing p.o.c, primitive optic chamber; or, orifice between the closing ridges; s.o.c, secondary optic chamber; ci, ci, ciliary body; l, rudimentary lens; R, retina. (After Lankester.)
Fig. 90.—Eye in A, Loligo; B, Helix or Limax; C, Nautilus: a.o.c, anterior optic chamber; c, cornea; int, integument; ir, iris; l, lens; l´, external portion of lens; op.n, optic nerve; op.g, optic ganglion; p.o.c, posterior optic chamber; r, retina. (After Grenacher.)
The developed eye in the dibranchiate Cephalopods consists of a transparent cornea, which may or may not be closed over the front of the lens. Behind the cornea is a narrow chamber (the anterior optic chamber) which is continued for three parts[184] round the whole circle of the eye, and into which project the front portion of the lens and the folds of the iris. Throughout its whole extent, the anterior optic chamber is lined by the integument, the portion of which on the inner side is the choroid. The lens is divided into an outer and inner segment by a thin membrane, and is supported by the ciliary body which forms a continuation of the retina. The main portion of the lens lies within the posterior optic chamber, at the back and sides of which is found the retina (Grenacher).
There can be no doubt that the Cephalopoda use their eyes to observe, but there is nothing to show that any other Mollusca use their eyes for this purpose, the sense of smell in their case largely taking the place of visual observation. Madame Jeannette Power once saw[286] the Octopus in her aquarium holding a fragment of rock in one of its arms, and watching a Pinna which was opening its valves. As soon as they were perfectly open, the Poulpe, with incredible address and promptitude, placed the stone between the valves, preventing the Pinna from closing again, upon which it set about devouring its victim. The next day the Poulpe was seen, after crushing some Tellina, to stretch himself down close by a Triton nodiferus, and watch it attentively. After four hours the Triton emerged from its shell, when the Octopus sprang upon it, and surrounded it with its arms.
Powers of Vision in Land Mollusca.—The Helicidae are undoubtedly very short-sighted. Seldom emerging from their retreats except in twilight and darkness, they are naturally myopic, and see better in a subdued than in a bright light. Experiment has shown that a Helix can perceive an object better at 6 centimetres distance in a weak light than at 4 or 5 millimetres in a strong one. Cyclostoma elegans and Paludina vivipara are comparatively long-sighted, perceiving objects at a distance of 20 to 30 centimetres.[287] The increased power of vision is due, in these two cases, to increased elaboration in the construction of the eye, Paludina possessing a large and almost[185] spherical lens, to which the vitreous humour closely adheres, while in Cyclostoma the lens is remarkably hard, and the aqueous humour very abundant. According to V. Willem,[288] the Pulmonata are very sensitive to the slightest movement of the air or jarring of the surface on which they crawl, but are so short-sighted as only to perceive a confused image of a large object at about 1 cm., and to distinguish the form of objects at not more than 1 or 2 mm. The senses of touch and smell are far more active than that of sight. A bean-pod enclosed in a narrow glass case and placed before a hungry snail was not noticed, but when taken out of the case and placed 8 cm. behind the snail, the latter at once turned towards it to devour it.
Some interesting experiments were conducted by the same author with the view of ascertaining whether snails avoid or court the light. He placed a number of species in different wooden boxes, which were divided into a light and a dark compartment, having previously well soaked the boxes in water to secure a humid atmosphere and surface, and so induce the snails to move about. The result showed that nearly all species have a marked predilection one way or the other, but not all in the same way. Helix aspersa, Arion empiricorum, six species of Limax, and three of Planorbis, are lovers of darkness, while H. nemoralis, Succinea putris, and two species of Limnaea are lovers of light. Physa fontinalis stands alone in being quite indifferent.
M. Willem endeavoured further to discover whether any of the Mollusca possessed ‘dermatoptic perception,’ or the faculty of perceiving variation of light by means of the skin alone. He accordingly repeated the above-mentioned experiments, having previously extirpated the eyes in all cases. The result was remarkable. In a few instances the experiment was not conclusive, but H. aspersa, A. empiricorum, several species of Limax, and one Limnaea shunned or sought the light just as they had done when their eyes were present. A few marine Mollusca (Littorina littorea, Trochus cinerarius, T. umbilicatus, Patella vulgata) were also shown to be exceedingly sensitive to the impact of a shadow, whether with or without their eyes.
Blind and Eyeless Mollusca.—In a large number of marine[186] Mollusca which habitually creep about half buried in wet sand (Bullia, Sigaretus, Scaphander, Philine), eyes are altogether absent. In some species of Natica and Sigaretus, and in Doris, eyes are developed, but are enclosed in a thick layer of skin, through which they can probably do little more than faintly appreciate different degrees of light and darkness. Chiton has cephalic eyes in the embryo, but loses them in the adult stage. The two great Auricula, A. auris Judae and A. auris Midae, which habitually creep about in the liquid mud of mangrove swamps, have entirely lost their eyes. Certain pelagic Mollusca seem to have a tendency, which is not easily explained, to lose their eyes or the power of seeing with them. Thus Ianthina has no eyes at all. Pteropoda as a rule have no eyes, and the few that have (Creseis, Cavolinia) possess only certain pigmented spots placed near to the nervous centres. In the Heteropoda, however, and the Cephalopoda, many of which are pelagic, the eyes are unusually large.
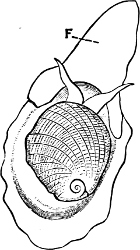
Fig. 91.—Sigaretus laevigatus Lam., a species frequenting wet sand, and destitute of external eyes: F, anterior portion of foot. (After Souleyet.)
Eyes in Deep-sea and Underground Mollusca.—Deep-sea Mollusca, as a rule, possess no visual organs, or possess them only in a rudimentary state, but this rule has its exceptions. Dr. Pelseneer found[289] no trace of eyes in two species of Pleurotoma from 1850 and 1950 fath., none in a Fossarus from 1400 f., none in a Puncturella from 1340 f. A remarkable form of Voluta (Guivillea) from 1600 f. possessed eyes which could hardly be functional, as they were destitute of pigment, and exhibited other changes of structure. On the other hand, it is remarkable to notice that in three different species of Trochus from 450 f., 565 f., and 1375 f., the eyes were pigmented and well developed.
In land Mollusca which live beneath the surface of the ground or in absolute darkness, the eyes are generally more or less modified. Thus in Testacella, which usually burrows deeply in the soil, but occasionally emerges into the open air, the eyes are very small, but distinct and pigmented. Our little Caecilianella[187] acicula, which is never seen above the surface, is altogether destitute of eyes. A species of Zospeum, a Helix, and a Bithynella from dark caves in Carniola have suffered a similar loss. On the other hand, a small Hyalinia from a dark cave in Utah (probably a recent addition to the cave fauna) has the eyes normally developed.
Eyes of Onchidium.—Many species of Onchidium, a naked land pulmonate which creeps on rocks near high-water mark, are provided with dorsal eyes of various degrees of organisation, and in numbers varying up to nearly one hundred. The tropical Onchidium are the prey of a fish (Periophthalmus) which skips along the beach by the aid of its large ventral fins, and feeds principally on insects and Onchidium. Karl Semper suggests[290] that the eyes are of service to Onchidium as enabling it to apprehend the shadow of the approaching Periophthalmus, and defend itself by suddenly contracting certain glands on the skin and expressing a liquid secretion which flies into the air like shot and frightens the Periophthalmus away. This theory for it is no more than theory—may or may not be true, but it is remarkable that Onchidium with dorsal eyes have precisely the same geographical distribution as Periophthalmus, and that where no Periophthalmus exists, e.g. on our own S.W. coasts, the Onchidium are entirely destitute of dorsal eyes. In those species of Onchidium which have no dorsal eyes, the latter are on the tips of the tentacles, as in Helix. The eyes are developed on the head, and afterwards ascend with the growth of the ommatophores, while in Helix the ommatophores are formed first, and the eyes developed upon them.[291]
Dorsal Eyes in the Chitonidae.—The remarkable discoveries of Moseley with regard to the dorsal eyes of Chiton were first published in 1884.[292] He happened to notice, while examining a specimen of Schizochiton incisus, a number of minute black dots on the outer surface of the shell, which appeared to refract light as if composed of glass or crystal. These ‘eyes,’ in all the species of Chiton yet examined, are restricted to the outer surface of the exposed area of the shell, never being on the laminae of insertion or on the girdle. In certain sub-genera of Chiton the eyes are scattered irregularly over the surface, in others[188] they are arranged symmetrically in rows diverging from the apex of each plate, but in old specimens the eyes towards the apices are generally rubbed off by erosion or abrasion. Moseley regarded the occurrence of scattered eyes as indicating an original stage of development, when the eyes were at first disposed irregularly all over the surface of the shell; the gathering into regular rows showing a later stage.
Fig. 92.—Dorsal eyes of Chitonidae, showing the various forms of arrangement in the first and fourth valves of 1, 1a, Acanthopleura spinigera Sowb., E. Indies, × 2; 2, 2a, Tonicia suezensis Reeve, Suez, × 3; 3, 3a, Acanthopleura granulata Gmel., W. Indies, × 2; 4, 4a, Tonicia lineolata Fremb., Chili, × 2. From specimens in the Museum of Zoology, Cambridge.
The eyes appear to be invariably more numerous on the anterior plate. Thus in Corephium aculeatum there are about 12,000 in all, of which more than 3000 are on the anterior plate. In Schizochiton they are arranged in very symmetrical rows, six of which are situated on the anterior, and only two, sometimes only one, on the central plates. In Tonicia marmorata the eyes are sunk in little cup-shaped depressions of the shell, possibly to escape abrasion. As regards shape and size, in Ch. incisus they are circular, and about 1/35 inch in diameter, this being the largest size known; in Ch. spiniger and Ch. aculeatus they are oval, measuring about 1/100 x 1/600 inch. There are no eyes in Chiton proper, nor in Mopalia, Maugeria,[189] Lorica, and Ischnochiton.[293] None of our English species appear to possess them.[294]
Eyes in Bivalve Mollusca.—Some, possibly most, of the Pelecypoda possess, in the larval state, true paired eyes at the oral end of the body. These become aborted as the animal develops, since that part of the body becomes entirely screened from the light by the growth of the shell. To compensate for their loss, numerous ocelli, or pigmented spots sensitive to the action of light, are in many cases developed on different parts of the mantle, functionally corresponding to the ‘eyes’ of Chiton described above. As in Chiton, too, we have here an interesting series of instances in which true eyes have suffered total obliteration, through disuse, and, as if to restore to the animal in some measure its lost sense, visual organs of a low power have subsequently been developed and are now observed in various stages of specialisation.
Concentration of Eyes in Special Parts of the Mantle.—Sharp has shown[295] that in several species of Ostrea, Cardium, Anomia, Lima, Avicula, Arca, and Tellina pigmented cells, with a highly refractive cuticle, are scattered over a considerable portion of the mantle. Experiment has proved the powers of ‘vision,’ i.e. of sensitiveness to different degrees of light, possessed by these organs. In Dreissena polymorpha, Tapes decussatus, and two species of Venus these cells are concentrated on that particular part of the mantle which is not always covered by the shell, i.e. the siphon, but since the siphon can be completely retracted within the shell, there is no special provision for their protection. A further step is shown in the case of Mya arenaria, where the siphon is scarcely capable of complete retraction. Here, while some of the pigment cells are scattered[190] about over the surface of the siphon, the majority are placed in grooves at the base of the siphonal tentacles, forming an intensely black band round them. A higher stage still is shown in Solen vagina, S. ensis, and Mactra solidissima, where the cells are situated only in the siphonal grooves, which are more or less specialised in numbers and complexity.
Arca Noae, according to Patten, is very sensitive to any sudden change in the amount of light falling upon its mantle-edge. A faint shadow cast upon it by the hand is sufficient to cause it to close its valves quickly, but always one or two seconds afterwards, the promptitude in all cases depending upon the depth of the shadow. Sensitiveness in this direction was found to depend greatly upon the vitality of the animals themselves, since it always became less in those specimens which had been kept for long in confinement. A shadow was not always necessary to make them close. An ordinary black pencil, if approached within two or three inches with extreme caution, produced the same result, while a glass rod brought within the same distance, and even moved rapidly to and fro, appeared to cause no alarm. Sensitiveness to change in intensity of light was experimentally noticed by the same author in the case of Ostrea, Mactra, Avicula (to a special extent), and Cardium. It is very remarkable to find that increased elaboration in the structure of the eyes does not necessarily carry with it increased sensitiveness, i.e. higher visual powers. Avicula, which is only provided with a few scattered ommatidia, which would entirely escape the notice of any one who had not seen them better developed elsewhere, was considerably more sensitive to light and shade than Arca, with its eyes of conspicuous size and much more perfect organisation, instantly contracting the mantle upon the impact of a shadow so faint as to be invisible to the experimenter.[296]
Visual Faculties of Solen and Ostrea.—The visual power of Solen may be exemplified by any one who is walking along almost any of our sandy bays at extreme low-water mark. If the day be warm and sunny, numbers of Solen will be seen raising themselves an inch or two out of their holes; but if you wish to catch them you must approach very cautiously, and on no account allow your shadow to fall upon them, or they will[191] pop down into their burrows in an instant, and it is vain to attempt to dig them out. ‘How sensitive,’ remarks Mr. W. Anderson Smith, with reference to oysters,[297] ‘the creatures are to the light above them; the shadow [of the boat] as it passes overhead is instantaneously noted, and, snap! the lips are firmly closed.’
Ocelli of Pecten.—In Pecten and Spondylus the ocelli are remarkably large and prominent, shining like precious stones, and are placed along the two edges of the mantle so as to receive the light when the shell gapes (Fig. 93). In Pecten opercularis, jacobaeus, and maximus their number varies from 80 to 120. In Spondylus gaederopus, a very inequivalve shell, 60 have been counted on the right or fixed valve, and 90 on the left or upper valve. Each ocellus is connected, by means of its optic nerve, with the large circumpalleal nerve, and so with the branchial ganglion. They possess a cornea, lens, choroidea, and optic nerve, and, according to Hickson,[298] bear a considerable resemblance to the vertebrate type of eye. In spite of this, the power of vision in these genera does not appear at all superior to that of other Pelecypoda.
Fig. 93.—Pecten opercularis L., showing the ocelli on the two edges of the mantle.
Fig. 94.—Compound eyes (c.e) of Arca barbata L.; m.l, mantle fold; omm, ommatidia. (After Patten.)
According to the elaborate investigations of Patten, the ‘eyes’ in Arca occur upon the middle or ‘ophthalmic’ fold of the mantle-edge, which is thickened at the end to admit of their reception. Along this is ranged a row of dark brown spots of various sizes, which are larger at the anterior and posterior ends of the mantle-edge, but smaller and more numerous towards the middle. These brown spots, or ‘eyes,’ are many of them compound, being made up of the fusion of a number of[192] ommatidia (from 10 to 80) into one large round eye, which is generally elevated above the surface of the surrounding epithelium. Sometimes these eyes themselves tend to fuse together. In one specimen of Arca Noae, 133 of these faceted eyes were counted in one mantle border, and 102 in the other.
There can be little doubt that the development of these functional eyes, or sensitive spots, in bivalve Mollusca, is due to special needs. They appear to be entirely absent in fresh-water bivalves (with the exception of Dreissensia, which is obviously a marine genus recently become fresh-water), while they are most abundant in genera living between tide marks (Solen, Mya, Mactra), and most highly specialised in a genus that is, for a bivalve, of singularly active habits (Pecten). Now genera living in sand between tide marks, as the three above-mentioned genera are in the habit of doing, and also protruding their siphons, and occasionally a considerable portion of their shells, out of their burrow, are manifestly very much at the mercy of their watchful enemies the gulls, and anything which would enable them to apprehend the approach of their enemies would be greatly to their advantage. Here, perhaps, lies the explanation of the greater elaboration of these pigmented spots in littoral genera, as compared with those inhabiting deeper water. Pecten, again, a genus distinguished by great activity, which can ‘fly’ for considerable distances in the water by flapping its valves together and expelling the water from the apertures at either side of the hinge, may be greatly assisted by its ocelli in directing its flight so as to escape its enemies.
The sense of smell—touch at a distance, as Moquin-Tandon has called it—is probably the most important sense which the Mollusca possess, and is unquestionably far more valuable to them than that of sight. Any one who has ever enjoyed the fun of hauling up lobster pots will recollect that part of the contents was generally a plentiful sprinkling of Buccinum, Nassa, and Natica, attracted by the smell of the stinking piece of fish with which the trap was baited. According to Mr. J. S. Gibbons,[299] Bullia rhodostoma congregates in hundreds on gigantic[193] medusae which are stranded on the sandy bays near the Cape of Good Hope. Dr. J. G. Jeffreys says[300] that quantities of the common Neptunea antiqua “are procured on the Cheshire coast by the fishermen placing a dead dog on the sands at low-water mark during spring tides. The bait is then completely covered with stones, which are piled up like a cairn. On the next turn of the tide the heap of stones is visited, and the whelks are found on the surface in great numbers, having been apparently attracted by the smell of the bait, but unable to get at it.” Mr. W. A. Lloyd kept specimens of Nassa reticulata in a tank in the sand, at the bottom of which they usually remained buried. If a piece of meat of any kind were drawn over the sand, the Nassa would appear above the surface in a few minutes. Half-picked beef or mutton bones, if placed in the tank, were covered in a few minutes. In fact, no animal matter, whether living or dead, could be introduced without the Nassa smelling it, and coming up to see what they could get.[301]
Any one can experiment for themselves on the olfactory powers of our common snails or slugs. Moquin-Tandon records[302] two interesting cases, one communicated to him by letter, the other occurring to himself. His correspondent, a M. Parenteau, was one day walking along a dusty high-road, when he noticed, near the middle of the road, an empty bean-pod and two Arions eating it. Attributing the meeting of feeders and food to mere chance, he was walking on, when he noticed a second bean-pod, and, about two yards away from it, a third Arion, hurrying straight towards it. When the Arion had yet more than a yard to traverse, M. Parenteau picked up the bean and put it in his pocket. The Arion stopped, raised its head, and turned in every direction, waving its tentacles, but without advancing. M. Parenteau then carried the bean to the other side of the road, and put it in a small hole behind a piece of stone. The Arion, after a moment’s indecision, started off straight for the bean. Again the position of the precious morsel was changed, and again the Arion made for it, this time without being further tantalised. M. Moquin-Tandon noticed, one rainy day in the botanical gardens at Toulouse, two Limax maximus approaching a rotten apple from different directions. He changed the position[194] of the apple several times, placing it at a sufficient distance, to be sure they could not see it, but they always hit it off correctly, after raising their heads and moving their long tentacles in every direction. It then occurred to him to hold the apple in the air, some centimetres above the head of the Limax. They perceived where it was, raised their heads and lengthened their necks, endeavouring to find some solid body on which to climb to their food.
Several of the land Mollusca have the power of exhaling a disagreeable smell, Hyalinia alliaria smelling strongly of garlic, and Stenogyra decollata of laudanum; but this need not be any argument for the sense of smell in the creatures themselves.
Position of Olfactory Organs in Pulmonata.—Most authorities are of opinion that the olfactory organs are situated in the tentacles. Moquin-Tandon considered that in the Helicidae and Limacidae the sense of smell is confined to the little knob or elevation at the end of the longer tentacles, close to the eye. He found that when he cut off these tentacles both in Limax and Arion, the creatures were quite unable to discover the whereabouts even of strongly-scented food. The same author believed that in the Basommatophora the sense of smell was present in the whole of the tentacle, which is covered with an exceedingly sensitive ciliated epithelium. Lacaze-Duthiers, however, places the olfactory sense in this group at the outer side of the base of the tentacles, near to the eyes. Some authorities[303] deny that the Helicidae have the olfactory organ at the tip of the tentacles, and locate it in a pedal gland near the mouth, which contains conspicuous sensitive cells. A Helix whose tentacles had been removed manifested its repulsion to the smell of spirits of turpentine, while another Helix, which was unmutilated, did not object to the turpentine being held between its tentacles. Altogether, then, the exact position of the smell-organ in the Helicidae must be considered as not yet thoroughly determined. Simroth holds that the sense of smell is distributed over the whole soft integument, and is especially concentrated in the feelers, and in the neighbourhood of the respiratory orifice.[304]
In nearly all marine Mollusca yet examined, the organ of smell or osphradium is in situation intimately connected with the breathing organs, being generally placed near their base,[195] with the object, apparently, of testing the quality of the water before it passes over the branchiae. It consists of a patch of the epithelium, modified in a special manner, and connected by its own nerve with one of the visceral ganglia.
An osphradium does not necessarily occur in all genera; for instance, it has not been detected in Fissurella. It is most highly specialised in the Conidae, and in the carnivorous Gasteropoda generally. In Buccinum undatum, for instance, it is very large indeed, and, from its plumed form, has sometimes been mistaken for an accessory branchia (Fig. 95). In Haliotis it is paired, one lying in close proximity to each of the two branchiae, but in Turbo it is single, corresponding to the single branchia. In Chiton there is an osphradium at the base of each separate gill filament, making a total of twenty or more on each side. Its position in Physa and in Cyclostoma will be seen by reference to Figs. 103 and 104 (p. 205). In the Pelecypoda the osphradia are paired, and lie adjacent to the posterior adductor muscle, close to the hinder end of the axis of the branchiae. In the Tetrabranchiate Cephalopoda there are two osphradia, placed between the bases of the two pairs of gills. In the Dibranchiates on the other hand, a groove above the eyes has been regarded as the seat of the organ of smell. This groove contains sensory and ciliated cells, and appears to be connected with a special nerve centre of its own, which ultimately is derived from the cerebral ganglion.
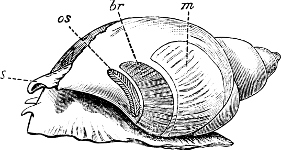
Fig. 95.—Buccinum undatum L., deprived of its shell, showing the relative position of branchia (br) and osphradium (os); m, mucous glands; s, siphon. The portion of the mantle covering the osphradium has been removed.
Scarcely any instances of the exercise of the sense of smell on the part of bivalve Mollusca have been recorded. Something of the sort, however, seems to have been present in a case related by Mr. R. L. King.[305] A skull of a fox had been placed in a small ditch in order to soak, and after a few days, when taken out, was found to be covered with Pisidium pusillum to the number of at least two hundred, which had been probably[196] attracted from the water in the immediate neighbourhood by the smell of the decaying flesh.
Experiments made with a view to ascertain whether the Mollusca are sensitive to noises have usually led to the conclusion that they are deaf to very loud sounds. This is the more curious, because an undoubted auditory apparatus has been discovered in a large number of genera. In the case of an experiment, it is not easy to be sure that the animal is not affected, at least in part, by the shock or jar, rather than by the actual sound. In some experiments, however, conducted at the Plymouth Marine Biological Laboratory, Mr. Bateson found[306] that Anomia could be made to shut its shell by smearing the glass of the tank with the finger in such a way as to make a creaking sound. It was evident that the cause of alarm was not the jarring of the solid framework of the tank, for the same result occurred when the object on which the Anomia were fixed was suspended in the water by a thread. It was found that the sound had to be of a particular pitch to excite the attention of the mollusc.
As a rule the organ of hearing is nothing more than a small vesicle or sac (the otocyst), filled with a fluid secretion, in which are suspended one or usually more calcareous concretions known as otoliths. By means of cilia, which connect with sense-cells, these otoliths are given a peculiar movement or oscillation in the medium in which they are suspended. The number of the otoliths varies in different genera and species; there are several hundreds in Arion and Limax, about a hundred in Helix pomatia, nemoralis, hispida, arbustorum, rotundata, Succinea putris, and Limnaea stagnalis; about fifty in Planorbis contortus and Physa fontinalis, only one in Cyclostoma elegans. The number increases with age. In young specimens of Limn. stagnalis as few as ten, nine, and seven have been noticed.[307]
The otocysts are always paired, and, in Gasteropoda, are placed close to the pedal ganglia. The acoustic nerve, however, has been shown by Lacaze-Duthiers to connect with the cerebral ganglia in certain cases. The otocysts are never on the surface[197] of the body and are rarely connected with it by any passage or tube; it is probable therefore that sound reaches them simply through the medium of the tissues.
In the Pelecypoda the otocyst is similarly situated near the pedal ganglion, and is probably (though this has not yet been proved) similarly connected with the cerebral. There is only a single otolith. Pelseneer finds[308] in Nuculidae alone a free communication between the otocyst and the exterior. Anodonta has been observed[309] to withdraw its foot into the shell at the noise of an opening door, a loud voice, or a shrill whistle, whether in a basin of water or lying on a study table.
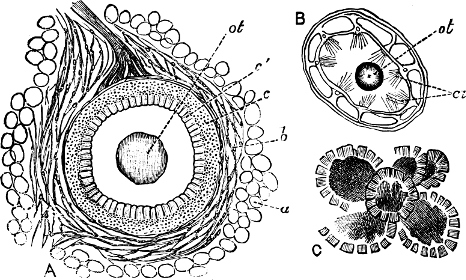
Fig. 96.—Illustrating the otocyst in A, Anodonta, B, Cyclas; ot, otolith; a, b, c, c´, cellular layers surrounding the chamber; ci, cilia on interior walls of chamber: C, an otolith crushed. (After Simroth.)
Delage extirpated the otocysts in certain Octopoda, and obtained some unexpected results. He found that remarkable effects were produced upon the animal’s powers of locomotion, so that it was unable to preserve its proper balance in the water when in rapid motion, but its body was forced to undergo a form of rotation more or less pronounced. He concluded that the otocysts must possess, besides their auditory functions, a power which stands in some relation to the proper orientation of the body in locomotion, a power which is not wholly supplied by sight and touch alone. The otocysts may thus regulate locomotion by stimulating muscular acts which tend to keep the body in the straight line during the process of movement.[310]
[198]
One of the most characteristic organs of the Mollusca is the foot, which, under one form or another, occurs throughout the whole phylum. The foot is a thickening, on the ventral side, of a portion of the integument of the animal, modified to serve different forms of motion. It attains its maximum relative area in the Chitonidae, many Nudibranchs, and the slugs generally, in nearly all of which there is no portion of the body which is not subtended by the foot. Here too it presents the form of a regular disc or ellipse, which is more or less produced. In many cases, however, the foot becomes modified in such a way that we are enabled to recognise well-marked anterior and posterior portions, which have received the name of propodium and metapodium respectively, while the intervening central portion is termed the mesopodium.
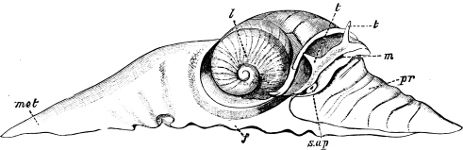
Fig. 97.—Sigaretus laevigatus Lam., showing excessive development of the propodium (pr) and metapodium (met) in a mollusc living in sand (the shell, which covers only the liver and adjacent parts, has been removed); l, liver; s.ap, aperture of proboscis, here deflected from the median line; t, t, tentacles. (After Quoy and Gaimard.)
The propodium is most strongly developed in genera which crawl about in wet sand, e.g. Natica, Sigaretus, Oliva, Harpa, Scaphander (Figs. 97 and 98, and compare Fig. 91). In such cases it seems to serve as a sort of fender or snow-plough, to push the sand away on both sides of the path the animal is traversing. In some species of Sigaretus the propodium becomes as it were banked up against the head and proboscis, which are thus unnaturally elevated, or tend to disappear altogether. Bullia (Fig. 62), which crawls about rapidly on wet sand, appears to attain its object by a wide extension of the foot on all sides, and so slides over the sand instead of ploughing[199] through it; the little lappets at the end of the ‘tail’ probably serve as rudders.
In Melampus and Pedipes the propodium is marked off by a groove across the ventral surface. When the animal is in motion it first advances the propodium and then pulls the rest of the foot after it with the looping gait of certain caterpillars. In many Cyclostomatidae this groove, instead of being transverse, is longitudinal, and the animal advances first the right and then the left segment of the foot, which gives it a swaying motion from side to side.
Upon the metapodium lies the operculum, when it occurs. As a rule the metapodium is not sharply marked off from the rest of the foot. In Strombus (Fig. 99) it becomes erected into a sort of hump or column, on the top of which the operculum is situated.
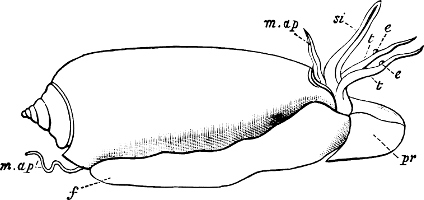
Fig. 98.—Oliva textilina Lam., showing how the front part of the foot (f) is developed into a sort of fender, the propodium (pr); e, e, eyes; m.ap, front appendage of mantle; m.ap´, hinder appendage of mantle, folded into the suture when the animal is at rest; si, siphon; t, t, tentacles. (After Quoy and Gaimard.)
The epipodium is a prominent fold or border, which occurs upon the upper edge of the foot in most Diotocardia. In Haliotis it is of considerable breadth, and is covered by a number of lobes which spring from a moss-like prolongation of the skin. From the epipodium are developed the lateral tentaculae of Monodonta (Fig. 82, p. 178), and of other sub-genera of the Trochidae.[311]
In the Opisthobranchiata the lateral edges of the foot (the parapodia) are frequently produced into broad folds or wing-like extensions, which in many cases tend to fold over the shell, and, in conjunction with the mantle, eventually imbed it altogether. By the wavy motion of the parapodia the animal is[200] enabled to progress through the water. The paired natatory lobes of the Pteropoda are simply the parapodia of the Tectibranchs modified for swimming purposes.
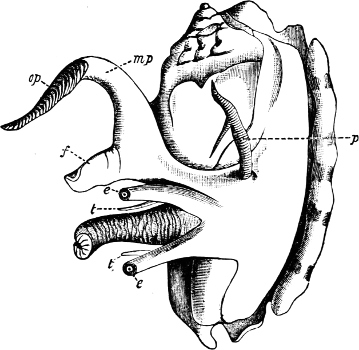
Fig. 99.—Strombus lentiginosus Lam., showing the modified form of the foot (f): e, e, eyes on their pedicels; mp, metapodium; op, operculum; p, penis; pr, proboscis; t, t, tentacles. (After Quoy and Gaimard.)
It is in the Heteropoda, Pteropoda, and most of all, the Cephalopoda, groups which have, for the most part, exchanged a crawling for a swimming life, that the modifications of the foot are most considerable. In Oxygyrus and Atlanta, for instance, the propodium and metapodium are sharply distinguished from the mesopodium, and no doubt have acquired, as a means of propulsion, the power of separate movement, the animal swimming with these portions of the foot uppermost. In Carinaria and Pterotrachea the metapodium has probably become continuous with the long axis of the body, while the so-called ‘foot’ with its sucker represents only the original propodium. In the Cephalopoda the arms and funnel represent the modified foot, the sides of which are prolonged into a number of very long specialised tentaculae. In the adult Cephalopod some of the arms have assumed a position in advance of the mouth, the latter being in fact surrounded by a circle of arms. But in the Cephalopod embryo the mouth opens as in the Gasteropoda, i.e. in advance of the arms, and it is only gradually that it becomes encircled by them. Arms and funnel alike are found to be innerved from the pedal ganglion.[312]
[201]
The pointed axe-shaped foot, which is characteristic of the majority of Pelecypoda, is doubtless derived from a form more akin to the flattened ‘sole’ of the Gasteropoda. A foot with something of this disc-shaped base actually occurs in some of the Nuculidae, the parapodia being furnished with pleats which recall similar formations in other Orders (Fig. 100). The principal modifications of the foot are due to its employment as a burrowing organ. In genera which burrow but slightly it is small and feebly developed, while in genera which habitually excavate, it becomes the largest and strongest organ of the body. At the same time it has a tendency to shift its position from the ventral to the anterior margin, accompanied by a corresponding narrowing of the shell, until it arrives at the position seen in Mollusca of the shape of Mya, Pholas, and Solen. In sedentary or attached genera, e.g. Pecten, Chama, Ostrea, the foot tends to become aborted.
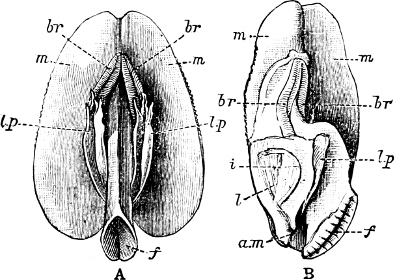
Fig. 100.—Yoldia limatula Say, Greenland, showing the short plumed branchiae (br, br), the gasteropodous foot (f), and the large labial palps (l.p, l.p): A, as seen from the ventral margin; B, from the left side, with the mantle turned back; a.m, position of anterior adductor muscle; i, intestine; l, liver; m, m, mantle.
The byssus gland, secreting a byssus of horny threads, is characteristic of many Pelecypoda, and may be observed by any one in the common mussel. It occurs in the larvae of many species which do not possess a byssus in the adult stage. The pedal gland of many Prosobranchiates, which secretes a tough and almost thread-like slime, is possibly homologous with the byssus gland of bivalves.
The Mollusca possess a nervous system, which usually consists of a number of nerve centres or ganglia, linked together by bands (the commissures) and sending out thread-like nerves which ramify into the various organs. The character of the[202] nervous system varies greatly in different groups, ranging as it does from a condition of extreme complexity, in which the ganglia are numerous and the commissures equally so, to that of considerable simplicity, in which ganglia are almost entirely absent.
The most important ganglia are (1) the cerebral,[313] which are always placed above or on either side of the mouth, and from which proceed the nerves of the eyes and tentacles; (2) the pedal, which in Gasteropoda are situated below the oesophagus, in Pelecypoda at the base of the foot, and from which the nerves of the foot and sometimes the acoustic nerve arise; (3) the pleural,[314] whose position varies considerably, but is always below the oesophagus and slightly above the pedal ganglia; these innervate the mantle, branchiae, heart, and viscera generally.
Gasteropoda.—The simplest form of nerve system as thus understood occurs in the Amphineura, and more particularly in the Chitons. Here we find four longitudinal nerve-cords, parallel to one another for nearly the whole length of the mollusc. The two exterior cords probably represent the pleural, the two interior the pedal nervous system. There being no head or tentacles, but simply a mouth at the anterior end, the cerebral ganglia do not exist, but they are represented by the curved ring formed by the massing together of the two nerve-cords on each side. The only distinct ganglia are a pair of buccal ganglia (which are developed on a pair of commissures which pass forward from the cerebral mass and innervate the lips and buccal region), and a much smaller group, the sublingual. The two pedal cords are united by a number of transverse parallel connectives, which recall similar modes of connexion in the Chaetopod worms and in Arthropoda.
This quadruple set of nerve-cords is characteristic of all the Amphineura, but the absence of ganglia is most marked in the Chitons. In Proneomenia and Neomenia there is a distinct cerebral ganglion, formed by the massing of the two ganglia into one, while in Proneomenia the lateral cords are joined to the pedal, as well as the pedal to one another, by connectives. In Chaetoderma the cerebral ganglia, though adjacent, are distinct, and both the pedal and lateral cords connect directly with them, while there are no transverse connectives.
[203]
The remaining three great divisions of Gasteropoda, namely, the Prosobranchiata, Opisthobranchiata, and Pulmonata, may be regarded as comprising two distinct types of nervous condition, according as the loop formed by the two visceral nerve-cords is twisted over itself, forming a figure of 8, or continues straight and uncrossed. In the former case, we get the condition known as streptoneurous, in the latter that as euthyneurous.[315] The Euthyneura include the whole of the Opisthobranchiata[316] and Pulmonata, the Streptoneura all the Prosobranchiata.
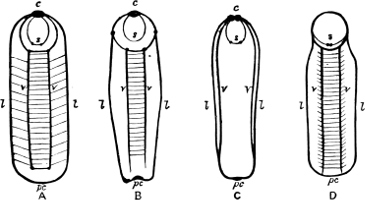
Fig. 101.—Nervous system of the Amphineura: A, Proneomenia; B, Neomenia; C, Chaetoderma; D, Chiton; c, cerebral ganglia; l, l, lateral cords; pc, posterior commissure; s, sublingual commissure or ring, with ganglia; v, v, pedal cords. (Alter Hubrecht.)
The simplest form of nervous system in the euthyneurous Gasteropoda occurs in the Opisthobranchiata. The cerebral, pleural, and pedal ganglia tend to become concentrated in a ring-like form, united by short commissures at the posterior end of the pharynx. The visceral loop is in some cases long, and the two or three visceral ganglia are then situated at its posterior extremity. The nervous system of the Pulmonata is of a similar type, the visceral loop being often much shorter, and tending to draw in towards the central group of ganglia. The tentacular and optic nerves are, as usual, derived from the cerebral ganglion, with which also the octocysts are probably connected by rather long nerves. A pair of buccal ganglia innervate the buccal mass, and are united by commissures with the right and[204] left cerebral ganglia. The osphradial nerve springs from one of the ganglia on the visceral loop, the osphradium itself being situated (in Limnaea) immediately above the pulmonary orifice and adjacent to the anus (Fig. 102). This massing of the ganglia is still better illustrated by the accompanying figure of Physa (Fig. 103), in which the animal is represented as if transparent, so that the ganglia and nerves are seen through the tissues.
Fig. 102.—I. Nervous system of Limnaea stagnalis L. The oesophagus has been cut and pulled forwards through the nerve-collar, so as to expose the lower surface of the buccal mass(dissected by F. B. Stead)
II. Right side of the head of Limnaea stagnalis. The overhanging flap of the mantle has been cut in the middle line, and the right half twisted back, so as to expose the pulmonary orifice, etc. The points A A on the mantle edge were continuous before the mantle was cut; the line BA is part of the free edge of the mantle.
An, anus; F, female generative orifice; J, portion of jaw; M, male generative orifice under right tentacle; Os, osphradium; P.O, pulmonary orifice.
Of the streptoneurous Gasteropoda, the nervous system of Fissurella and Haliotis shows distinct points of similarity to that of the Amphineura. The pedal nerves are united by transverse commissures throughout their entire length, while a double commissure unites the cerebral ganglia to the mass from which the pedal nerves proceed. In the great majority of the Streptoneura the ganglia (except the visceral) are more concentrated and the commissures are consequently much shorter. The accompanying figure of Cyclostoma, in which the animal is represented as in[205] that of Physa just described, illustrates this grouping of the ganglia, the twist of the visceral loop, and the position of the visceral ganglia at its posterior end.
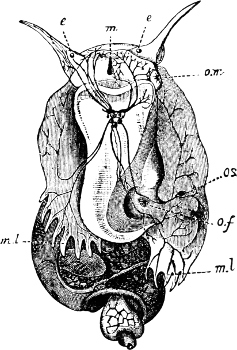
Fig. 103.—Nervous system of Physa acuta Drap., showing the massing of the ganglia at the hinder end of the pharynx: e, e, eyes; m, mouth; m.l, m.l, mantle lappets; o.f, female generative orifice; o.m, male generative orifice; os, osphradium. (After Lacaze-Duthiers.)
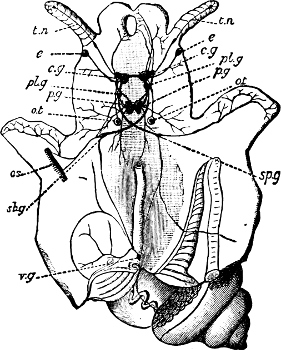
Fig. 104.—Example of a streptoneurous Gasteropod (Cyclostoma elegans Drap.): c.g, c.g, cerebral ganglia; e, e, eyes; os, osphradium; ot, ot, otocysts; p.g, p.g, pedal ganglia; pl.g, pl.g, pleural ganglia; sp.g, supra-intestinal ganglion; sb.g, sub-intestinal ganglion; t.n, tentacle nerve; v.g, visceral ganglion. (After Lacaze-Duthiers.)
Scaphopoda.—In the Scaphopoda the nervous system resembles that of the Pelecypoda. The cerebral and pleural ganglia lie close together, while the pedal ganglia are placed in the anterior part of the foot, connected with the cerebral ganglia by long commissures; the visceral loop is rather long, and the two visceral ganglia are adjacent to the anus.
Pelecypoda.—The nervous system in the Pelecypoda is the simplest type in which well-marked ganglionic centres occur. The ganglia are few, symmetrically placed, and are usually at a considerable distance apart. There are, as a rule, three distinct pairs of ganglia, the cerebral (cerebro-pleural), pedal, and visceral. The cerebral are formed by the fusion of the cerebral and pleural ganglia, which, however, in some cases (Protobranchiata) continue distinct.[317] They lie above or on each side of the mouth,[206] united by a commissure of varying length. Another pair of commissures unites them with the pedal ganglia, which are placed at the base of the foot, and are usually very close together, sometimes (as in Anodonta) becoming partially fused. The length of these commissures depends upon the distance between mouth and foot; thus they are very long in Mya and Modiola, and very short in Pecten. In cases where the foot is rudimentary or becomes aborted through disuse (e.g. Ostrea), the pedal ganglia may dwindle or disappear altogether. The visceral ganglia are on the ventral side of the posterior adductor muscle, beneath the rectum, and innervate the branchiae, osphradia, and the whole of the visceral sac. A pair of cerebro-visceral commissures traverses the base of the foot, surrounding it with a comparatively short loop (compare Fig. 106, c.v.c´), while a long commissure, which runs round the entire edge of the mantle, and supplies branching nerves to the mantle border and siphons (Fig. 106, c.v.c), may also connect the visceral and cerebral ganglia.
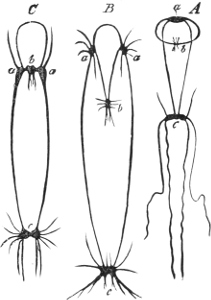
Fig. 105.—Nervous system of Pelecypoda: A, Teredo; B, Anodonta; C, Pecten; a, a, cerebral ganglia; b, pedal ganglia; c, visceral ganglia. (After Gegenbaur.)
Cephalopoda.—In the Cephalopoda the concentration of ganglia attains its maximum, and may perhaps be regarded as approaching the point at which a definite brain may be said to exist. Another point of distinction is the formation of special small ganglia upon the nerve-cords in different parts of the body. In the Tetrabranchiata (Nautilus) the cerebral and pedal ganglia form a broad ring which surrounds the oesophagus, the former giving out the optic nerves, with their special optic ganglion, and a pair each of buccal and pharyngeal ganglia, the latter the nerves of the arms and funnel. The visceral loop is still present in the form of a separate band, which innervates the branchiae,[207] osphradia, and viscera generally, forming a special genital ganglion in connexion with the reproductive organs. The principal ganglia of the Dibranchiata are still more concentrated, even the visceral loop being possibly united with the rest in forming an unbroken mass in which scarcely any trace of commissures can be detected. The pedal ganglion becomes separated into two portions, one of which innervates the arms, the other the funnel. Two peculiar ganglia (the stellate ganglia) supply a number of branching nerves to the mantle.
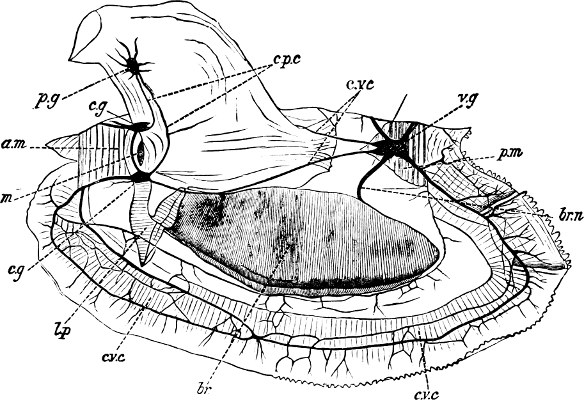
Fig. 106.—Nervous system of Cardium edule L.: a.m, anterior adductor muscle; br, branchiae; br.n, branchial nerve; c.g, c.g, cerebral ganglia; c.p.c, cerebro-pedal commissure; c.v.c’, cerebro-visceral commissure; c.v.c, cerebro-visceral commissure of mantle; l.p, labial palps: m, mouth; p.g, pedal ganglion; p.m, posterior adductor muscle; v.g, visceral ganglion. (After Drost, × 3.)
E. L. Bouvier, Système nerveux, morphologie générale et classification des Gastéropodes prosobranches: Ann. Sc. Nat. Zool. (7), iii. 1887, pp. 1–510.
J. Brock, Zur Neurologie der Prosobranchier: Zeit. wiss. Zool. xlviii. 1889, pp. 67–83.
O. Bütschli, Bemerkungen über die wahrscheinliche Herleitung der Asymmetrie der Gasteropoda, etc.: Morph. Jahrb. xii. 1886, pp. 202–222.
B. Haller, Zur Kenntniss der Muriciden. I. Anatomie des Nervensystems: Denksch. Math. Nat. Kl. Ak. Wien, xlv. 1882, pp. 87–106.
„ Untersuchungen über marine Rhipidoglossen. II. Textur des„ Centralnervensystems und seiner Hüllen: Morph. Jahrb. xi. 1885, pp. 319–436.
[208]
H. Grenadier, Abhandlungen zur vergleichenden Anatomie des Auges: Abh. Naturf. Gesell. Halle, xvi. 1884, pp. 207–256; xvii. 1886, pp. 1–64.
A. P. Henchman, The Origin and Development of the Central Nervous System in Limax maximus: Bull. Mus. C. Z. Harv. xx. 1890, pp. 169–208.
V. Hensen, Ueber das Auge einiger Cephalophoren: Zeit. wiss. Zool. xv. 1865, pp. 157–242.
C. Hilger, Beiträge zur Kenntniss des Gasteropodenauges: Morph. Jahrb. x. 1885, pp. 352–371.
Lacaze-Duthiers, Otocystes ou Capsules auditives des Mollusques (Gastéropodes): Arch. Zool. Exp. Gén. i. 1872, pp. 97–166.
„ „ Du système nerveux des Mollusques gastéropodes pulmonés aquatiques: ibid. pp. 437–500.
P. Pelseneer, Recherches sur le système nerveux des Ptéropodes: Arch. Biol. vii. 1887, pp. 93–130.
„ Sur la valeur morphologique des bras et la composition du système nerveux central des Cephalopodes: Arch. Biol. viii. 1888, pp. 723–756.
H. Simroth, Ueber die Sinneswerkzeuge unserer einheimische Weichthiere: Zeit. wiss. Zool. xxvi. 1876, pp. 227–348.
J. W. Spengel, Die Geruchsorgane und das Nervensystem der Mollusken: Zeit. wiss. Zool. xxxv. 1881, pp. 333–383.
[209]
The digestive tract, or, as it is often termed, the alimentary canal or gut, is a very important feature of the Mollusca. It may be regarded as consisting of the following parts: (1) a mouth or oral aperture: (2) a throat or pharynx; (3) an oesophagus, leading into (4) a stomach, (5) an intestine and rectum, ending in (6) an anus.
The primitive positions of mouth and anus were presumably at the anterior and posterior ends of the animal, as in the Amphineura and symmetrical Mollusca generally. But the modifications of original molluscan symmetry, which have already been referred to (p. 154, compare pp. 245, 246), have resulted in the anus becoming, in the great majority of Gasteropoda, twisted forward, and occupying a position on some point in the right side in dextral, and in the left in sinistral species.
The process of digestion, as the food passes from one end of the tract to the other, is performed by the aid of the secretions of various glands, which open into the alimentary canal at different points in its course. The principal of these are the salivary glands, situated on the pharynx and oesophagus, and the liver, biliary or hepatic gland, connecting with the stomach. With these may be considered the anal and ink-glands, which, in certain genera, connect with the terminal portion of the rectum.
1. The mouth is generally, as in the common snail and periwinkle, placed on the lower part of the head, and may be either a mere aperture, circular or semicircular, in the head-mass, or, as is more usual, may be carried on a blunt snout (compare Fig. 6, p. 10, and Fig. 68, p. 159), which is capable of varying degrees of protrusion. From the retractile snout has doubtless been[210] derived the long proboscis which is so prominent a feature of many genera (compare Figs. 1, B, and 99), and in some (e.g. Mitra, Dolium) attains a length exceeding that of the whole body. As a rule, Mollusca provided with a proboscis are carnivorous, while those whose mouth is on the surface of the head are Vegetable feeders, but this rule is by no means invariable. The mouth is thickened round the aperture into ‘lips,’ which are often extensile, and appear capable of closing upon and grasping the food. In the Pelecypoda the mouth is furnished, on each side, with a pair of special external lobes, the ‘labial palps,’ which appear to be of a highly sensitive nature, and whose object it is to collect, and possibly to taste, the food before it passes into the mouth.
2. The Pharynx, Jaws, and Radula.—Immediately behind the lips the mouth opens into the muscular throat, pharynx, or buccal mass. The pharynx of the Glossophora, i.e. of the Gasteropoda, Scaphopoda, and Cephalopoda, is distinguished from that of the Pelecypoda,[318] by the possession of two very characteristic organs for the rasping or trituration of food before it reaches the oesophagus and stomach. These are (a) the jaw or jaws, and (b) the radula,[319] odontophore, or lingual ribbon. The jaws bite the food, the radula tears it up small before it passes into the stomach to undergo digestion. The jaws are not set with teeth like our own; roughly speaking, the best idea of the relations of the molluscan jaw and radula may be obtained by imagining our own teeth removed from our jaws and set in parallel rows along a greatly prolonged tongue.[320]
In nearly all land Pulmonata the jaw is single, and is placed behind the upper lip. If a common Helix aspersa be observed crawling up the inside of a glass jar, or feeding on some succulent leaf, the position and action of the jaw can be readily discerned. It shows very black when the creature opens its mouth, and under its operation the edge of a lettuce leaf shows a regular series of little curved indentations, in shape not unlike the semicircular[211] bites inflicted by a schoolboy upon his bread and butter. The jaw of Helix (Fig. 107, B) is arched in shape, and is strengthened by a number of projecting vertical ribs. That of Limax (A) is straighter, and is slightly striated, without vertical ribs. In Bulimulus (C) the arch of the jaw is very conspicuous, and the upper edges are always denticulated; in Orthalicus there is a central triangular plate with a number of overlapping plates on either side; in Succinea (E) there is a large square accessory plate above the jaw proper. The form of the jaw is peculiar not only to the genus but to the species as well. Thus the jaw of H. aspersa is specifically distinct from that of H. pomatia, and that of H. nemoralis is distinct from both. Wiegmann has observed[321] that in young Arion, Limax, and Helix, the jaw consists of two pieces, which coalesce by fusion in the adult, thus indicating a stage of development in advance of the double jaw which is found in most of the non-pulmonate Mollusca. In all fresh-water Pulmonata there are two small accessory side plates besides the jaw proper (Fig. 107, F).
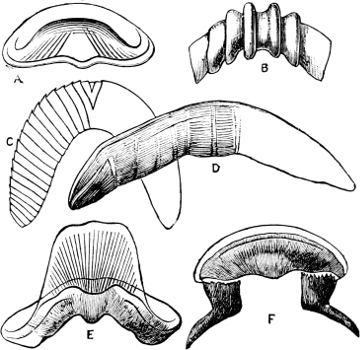
Fig. 107.—Jaws of various Pulmonata: A, Limax (gagates Drap., Lancashire, × 15); B, Helix (acutissima Lam., Jamaica, × 15); C, Bulimulus (depictus Reeve, Venezuela, × 20); D, Achatina (fulica Fér., Mauritius, × 7); E, Succinea (elegans Riss., Aral District, × 30); F, Limnaea (stagnalis L., Cambridge, × 30).
Nearly all the non-carnivorous Prosobranchiata, land, fresh-water, and marine alike, are provided with two large lateral jaws.[212] Many of these are sculptured with the most elaborate patterns, and appear to be furnished with raised teeth, like a file. In the Nudibranchiata the jaws are of great size and beauty of ornamentation (Fig. 109).
Fig. 108.—Jaws of A, Triton australis Lam., Sydney; B, Ampullaria fasciata Reeve, Demerara; C, Calliostoma punctulatum Mart., New Zealand; D, Cyclophorus atramentarius Sowb., Sanghir; all × 15.
Fig. 109.—Jaws of A, Chromodoris gracilis Iher., × 15; B, Scyllaea pelagica L., × 7; C, Pleurobranchus plumula Mont., × 10; D, Pleurobranchaea Meckelii Lam., × 5/2.
[213]
The carnivorous genera, whether marine (e.g. Conus, Murex, Buccinum, Nassa) or land (e.g. Testacella, Glandina, Streptaxis, Ennea), are entirely destitute of jaws, the reason probably being that in all these cases the teeth of the radula are sufficiently powerful to do the work of tearing up the food without the aid of a masticatory organ as well. Jaws are also wanting in the Heteropoda, and in many of the Nudibranchiata and Tectibranchiata.
In the Cephalopoda the jaws, or ‘beaks,’ as they are called, are most formidable weapons of attack. In shape they closely resemble the beaks of a parrot, but the hook on the dorsal side of the mouth does not, as in birds, close over the lower hook, but fits under it. Powerful muscles govern these mandibles, which must operate with immense effect upon their prey (Fig. 110).
Fig. 110.—Jaws of Sepia: A, in situ within the buccal mass, several of the arms having been cut away; B, removed from the mouth and slightly enlarged.
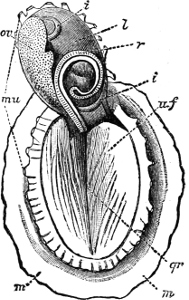
Fig. 111.—Patella vulgata L., showing the normal position of the radula, which is doubled back in a bow; the shell has been removed, and the whole visceral mass is turned forward, exposing the dorsal surface of the muscular foot: gr, longitudinal groove on this surface; i, i, intestine; l, liver; m, m, mantle edge; mu, muscles (cut through) fastening the visceral mass to the upper sides of the foot; ov, ovary; r, radula; u.f, upper or dorsal surface of the foot.
The Radula.[322]—When the food has passed beyond the operation[214] of the jaw, it comes within the province of the radula, the front part of which perhaps co-operates to a certain extent with the jaw in performing the biting process. The function of the[215] radula as a whole is to tear or scratch, not to bite; the food passes over it and is carded small, the effect being very much the same as if, instead of dragging a harrow over the surface of a field, we were to turn the harrow points upwards, and then drag the field over the harrow.
The radula itself is a band or ribbon of varying length and breadth, formed of chitin, generally almost transparent, sometimes beautifully coloured, especially at the front end, with red or yellow.[323] It lies enveloped in a kind of membrane, in the floor of the mouth and throat, being quite flat in the forward part, but usually curving up so as to line the sides of the throat farther back, and in some cases eventually forming almost a tube. The upper surface, i.e. the surface over which the food passes, is covered with teeth of the most varied shape, size, number, and disposition, which are almost invariably arranged in symmetrical rows. These teeth are attached to the cartilage on which they work by muscles which serve to erect or depress them; probably also the radula as a whole can be given a forward or backward motion, so as to rasp or card the substances which pass over it.
The teeth on the front part of the radula are often much worn (Fig. 112), and probably fall away by degrees, their place being taken by others successively pushed up from behind. At the extreme hinder end of the radula the teeth are in a nascent condition, and there are often as many as a dozen or more scarcely developed rows. Here, too, lie the cells from which the teeth are originally formed.
The length and breadth of the radula vary greatly in different genera. In Littorina it is very narrow, and several times the length of the whole animal. It is kept coiled away like a watch-spring at the back of the throat, only a small proportion of the whole being in use. I have counted as many as 480 rows in the common Littorina littorea. In Patella it is often longer than the shell itself, and if the radula of a large specimen be freshly extracted and drawn across the hand, the action of the hooks can be plainly felt. In Aerope, the Turbinidae generally, and Haliotis it is very large. In Turritella, Aporrhais, Cylichna,[216] Struthiolaria, and the Cephalopoda it is small in proportion to the size of the animal. In the Pulmonata generally it is very broad, the length not exceeding, as a rule, thrice the breadth; in most other groups the breadth is inconsiderable, as compared to the length.
The Radula is wanting in two families of Prosobranchiata, the Eulimidae and Pyramidellidae, which are consequently grouped together as the section Gymnoglossa. It is probable that in these cases the radula has aborted through disuse, the animals having taken to a food which does not require trituration. Thus several genera contained in both these families are known to live parasitically upon various animals—Holothurians, Echinoderms, etc.—nourishing themselves on the juices of their host. In some cases, the development of a special suctorial proboscis compensates for the loss of radula (see pp. 76–77). In Harpa there is no radula in the adult, though it is present in the young form. No explanation of this fact has yet been given. It is also absent in the Coralliophilidae, a family closely akin to Purpura, but invariably parasitic on corals, and probably nourished by their exudations. There is no radula in Entoconcha, an obscure form parasitic on the blood-vessels of Synapta, or in Neomenia, a genus of very low organisation, or in the Tethyidae, or sea-hares, or in one or two other genera of Nudibranchiata.
Fig. 112.—Example of a front portion of a radula (Cantharus ringens Reeve, Panama), much worn by use. × 70.
The number of teeth in the radula varies greatly. When the teeth are very large, they are usually few in number, when small, they are very numerous. In the carnivorous forms, as a rule, the teeth are comparatively few and powerful, while in the phytophagous genera they are many and small. Large hooked and sickle-shaped teeth, sometimes furnished with barbs like an arrow-head, and poison-glands, are characteristic of genera which feed on flesh; vegetable feeders, on the contrary, have the teeth rounded, and blunter at the apex, or, if long and narrow, so slender as to be of comparatively little effect.[217] Genera which are normally vegetarian, but which will, upon occasion, eat flesh, e.g. Limax and Hyalinia, exhibit a form of teeth intermediate between these two extremes (see Fig. 140, A).
In Chaetoderma there is but one tooth. In Aeolis coronata there are about 17, in A. papillosa and Elysia viridis about 19, in Glaucus atlanticus about 21, in Fiona nobilis about 28. In the common whelk (Buccinum undatum) there are from 220 to 250, in the common periwinkle about 3500. As many as 8343 have been counted in Limnaea stagnalis, about 15,000 in Helix aspersa (that is, about 400,000 to the square inch), about 30,000 in Limax maximus, and as many as 40,000 in Helix Ghiesbreghti, a large species from Mexico; they are very numerous also in Nanina, Vitrina, Gadinia, and Actaeon. But Umbrella stands far and away the first, as far as number of teeth is concerned. In both U. mediterranea and U. indica they entirely baffle calculation, possibly 750,000 may be somewhere near the truth.
The teeth on the radula are almost invariably disposed in a kind of pattern, exactly like the longitudinal rows of colour in a piece of ribbon, down the centre of which runs a narrow stripe, and every band of colour on one side is repeated in the same relative position on the other side. The middle tooth of each row—the rows being counted across the radula, not longitudinally—is called the central or rachidian tooth; the teeth next adjacent on each side are known as the laterals, while the outermost are styled uncini or marginals. As a rule, the distinction between the laterals and marginals is fairly well indicated, but in the Helicidae and some of the Nudibranchiata it is not easy to perceive, and in these cases there is a very gradual passage from one set to the other.
The central tooth is nearly always present. It is wanting in certain groups of Opisthobranchiata, some of the carnivorous Pulmonata, and in the Conidae and Terebridae, which have lost the laterals as well. Voluta has lost both laterals and marginals in most of the species, and the same is the case with Harpa. In Aeolis, Elysia, and some other Nudibranchiata the radula consists of a single central row. Other peculiarities will be described below in their proper order.
The extreme importance of a study of the radula depends upon the fact, that in each species, and a fortiori in each genus[218] and family, the radula is characteristic. In closely allied species the differences exhibited are naturally but slight, but in well-marked species the differences are considerable. The radula, therefore, serves as a test for the distinction of genera and species. For instance, in the four known recent genera of the family Strombidae, viz. Strombus, Pteroceras, Rostellaria, and Terebellum, the radula is of the same general type throughout, but with distinct modifications for each genus; and the same is true, though to a lesser extent, for all the species hitherto examined in each of the genera. These facts are true for all known genera, differences of the radula corresponding to and emphasising those other differences which have caused genera to be constituted. The radula therefore forms a basis of classification, and it is found especially useful in this respect in dealing with the largest class of all, the Gasteropoda, and particularly with the chief section of this order, the Prosobranchiata. Thus we have—
| Prosobranchiata | Monotocardia | (a) Toxoglossa |
| (b) Rachiglossa | ||
| (c) Taenioglossa | ||
| (d) Ptenoglossa | ||
| (e) Gymnoglossa | ||
| Diotocardia | (f) Rhipidonlossa | |
| (g) Docoglossa[324] |
(a) Toxoglossa.—Only three families, Terebridae, Conidae, and Cancellariidae, belong to this section. There is no central tooth, and no laterals, the radula consisting simply of large marginals on each side. In Conus these are of great size, with a blunt base which contains a poison-gland (see p. 66), the contents of which are carried to the point by a duct. The point is always singly and sometimes doubly barbed (Fig. 116). When extracted, the teeth resemble a small sheaf of arrows (Figs. 113, 115). A remarkable form of radula, belonging to Spirotropis (a sub-genus of Drillia, one of the Conidae), enables us to explain the true history of the radula in the Toxoglossa. Here there are five teeth in a row, a central tooth, and one lateral and one marginal on each side, the marginals being very similar in shape to the characteristic shafts of the Conidae (Fig. 114). It is evident, then, that the great mass of the Toxoglossa have lost[219] both their central and lateral teeth, and that those which remain are true uncini or marginals. Spirotropis appears to be the solitary survival of a group retaining the primitive form of radula.
Fig. 113.—Radula of Bela turricula Mont. × 70.
Fig. 114.—Portion of radula of Spirotropis carinata Phil., Norway. × 70.
Fig. 115.—Eight teeth from the radula of Terebra caerulescens Lam. × 60.
The arrangement of teeth in all these sections is expressed by a
formula applicable to each transverse row of the series. The central
tooth, if present, is represented by 1, and the laterals and marginals,
according to their number, on each side of the central figure. Thus
the typical formula of the Toxoglossa is 1.0.0.0.1, the middle 0
standing for the central tooth which is absent, and the 0 on each
side of it for the absent laterals; the 1 on each extreme represents
the one uncinus in each row. Thus the formula for Spirotropis,
which has also one lateral on each side and a rachidian or central
tooth, is 1.1.1.1.1. Often the formula is given thus: 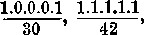 where 30 and 42 stand for the average
number of rows of teeth in Conus and Spirotropis
respectively; the same is sometimes expressed thus: 1.0.0.0.1 × 30;
1.1.1.1.1 × 42.
where 30 and 42 stand for the average
number of rows of teeth in Conus and Spirotropis
respectively; the same is sometimes expressed thus: 1.0.0.0.1 × 30;
1.1.1.1.1 × 42.
[220]
Fig. 116.—A tooth from the radula of Conus imperialis L., S. Pacific, × 50, showing barb and poison duct.
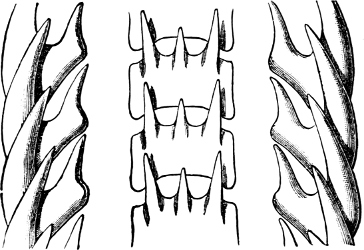
Fig. 117.—Portion of the radula of Melongena vespertilio Lam., Ceylon. × 30.
Fig. 118.—Portion of the radula of Eburna japonica Sowb., China. × 30.
Fig. 119.—Portion of the radula of Murex regius Lam., Panama. × 60.
(b) The Rachiglossa comprise the 12 families Olividae, Harpidae, Marginellidae, Volutidae, Mitridae, Fasciolariidae, Turbinellidae, Buccinidae, Nassidae, Columbellidae, Muricidae, and Coralliophilidae. Certainly most and probably all of these families are or have been carnivorous, the Coralliophilidae being a degraded group which have become parasitic on corals, and have lost their teeth in consequence. The characteristics of the[221] group are the possession of a central tooth with from one cusp (Boreofusus) to about fourteen (Bullia), and a single lateral more or less cuspidate, the outer cusp of all being generally much the largest. Thus in Melongena respertilio (Fig. 117) the central tooth is tricuspid, the central cusp being the smallest, while the laterals are bicuspid; in Eburna japonica (Fig. 118) the central tooth is 5-cusped, the two outer cusps being much the smallest. The teeth, on the whole, are sharp and hooked, with a broad base and formidable cutting edge. In the Olividae, Turricula, Buccinopsis, and the Muricidae the laterals are unicuspid and somewhat degraded (Fig. 119). In Mitra and the Fasciolariidae they are very broad and finely equally toothed like a comb (Figs. 120, 121). The whole group is destitute of marginals.
Fig. 120.—Portion of the radula of Imbricaria marmorata Swains. × 80.
Fig. 121.—Three rows of teeth from the radula of Fasciolaria trapezium Lam. × 40.
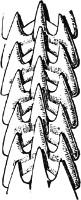
Fig. 122.—Six teeth from the radula of Cymbium diadema Lam., Torres Strait. × 25.
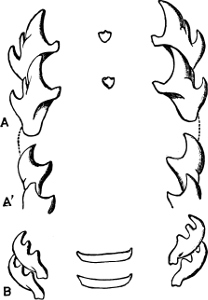
Fig. 123.—Examples of degraded forms of radula: A, Cantharus pagodus Reeve, Panama (nascent end), × 40; A´, same radula, central and front portion; B, Columbella varia Sowb., Panama, × 50.
Fig. 124.—Three rows of the radula of Sistrum spectrum Reeve, Tonga, × 80. The laterals to the right are not drawn in.
Several remarkable peculiarities occur. Harpa loses the radula altogether in the adult. In the young it has lost only the laterals, and consists of nothing but the central tooth. Marginella has no laterals; the central tooth is small and comb-shaped, with blunt cusps. In Voluta the laterals are generally lost, but in Volutomitra and one species of Voluta[325] they are retained. The central tooth usually has three strong cusps,[222] and is very thick and coloured a deep red or orange (Fig. 122); in the sub-genus Amoria it is unicuspid, in shape rather like a spear-head with broadened wings; in Volutolyria it is of a different type, with numerous unequal denticulations, something like the laterals of Mitra or Fasciolaria. Of the Mitridae, Cylindromitra has lost the laterals. Among the Buccinidae, Buccinopsis possesses a curiously degraded radula, the central tooth having no cusps, but being reduced to a thin basal plate, while the laterals are also weakened. This degradation from the type is a remarkable feature among radulae, and appears to be characteristic, sometimes of a whole family, e.g. the Columbellidae (Fig. 123, B), sometimes of a genus, sometimes again of a single species. Thus in Cantharus (a sub-genus of Buccinum) the radula is typical in the great majority of species, but in C. pagodus Reeve, a large and well-grown species, it is most remarkably degraded, both in the central and lateral teeth (Fig. 123, A). This circumstance is the more singular since C. pagodus lives at Panama side by side with C. ringeus and C. insignis, both of which have perfectly typical radulae. It is probable that the nature of the food has something to do with the phenomenon. Thus Sistrum spectrum Reeve was found to possess a very aberrant radula, not of the common muricoid[223] type, but with very long reed-like laterals. This singularity was a standing puzzle to the present writer, until he was fortunate enough to discover that S. spectrum, unlike all other species of Sistrum, lives exclusively on a branching coral.
The dental formula for the Rachiglossa is thus 1.1.1, except in those cases where the laterals are absent, when it is 0.1.0.
Fig. 125.—Portion of the radula of Cassis sulcosa Born., × 40. The marginals to the right are not fully drawn.
(c) The Taenioglossa comprise 46 families in all, of which the most important are Tritonidae, Cassididae, Cypraeidae, Strombidae, Cerithiidae, Turritellidae, Melaniidae, Littorinidae, Rissoidae, Paludinidae, Ampullariidae, Cyclophoridae, Cyclostomatidae, and Naticidae. The radula is characterised by a central tooth of very variable form, the prevailing type being multicuspid, the central cusp the largest, on a rather broad base; a single lateral, which is often a broad plate, more or less cusped, and two uncini, rather narrow, with single hooks, or slightly cusped. The accompanying figures of Cassis, Vermetus, and Cypraea, and those of Littorina and Cyclophorus given on pp. 20, 21, are good examples of typical taenioglossate radulae.
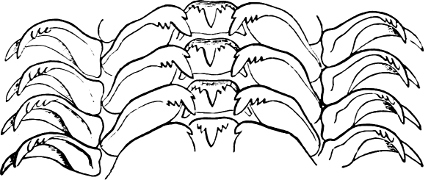
Fig. 126.—Four rows of teeth from the radula of Vermetus grandis Gray, Andamans. × 40.
In Homalogyra the radula is much degraded, the central tooth is large and triangular on a broad base, the lateral is represented only by a thin oblong plate, and the uncini are absent. In some species of Jeffreysia the uncini are said to be absent, while present in others. Lamellaria has lost both its uncini, but the radula of the allied Velutina is quite typical. A peculiar feature in this group is the tendency of the marginals to increase in number. A stage in this direction is perhaps[224] seen in Ovula, Pedicularia, and the Cyclostomatidae. Here the outermost of the two marginals is by far the larger and broader, and is strongly pectinated on its upper edge; in the Cyclostomatidae the pectinations are rather superficial; in Ovula (where both marginals are pectinated) they are decidedly deeper; in Pedicularia they are deeper still, and make long slits in the tooth, tending to subdivide it altogether. In Turritella the number of marginals is said to vary from none (in T. acicula) to three (T. triplicata), but the fact wants confirmation. Solarium is an aberrant form, possessing simply a number of long uncini, which recall those of Conus or Pleurotoma, and is therefore hard to classify; the allied Torinia has a radula which appears allied to Ovula or Pedicularia. In Triforis the teeth are identical throughout, very small, about 27 in a row, tricuspid on a square base, cusps short.
The normal formula of the Taenioglossa is 2.1.1.1.2; in Lamellaria, 1.1.1; in Triforis, 13.1.13, or thereabouts.
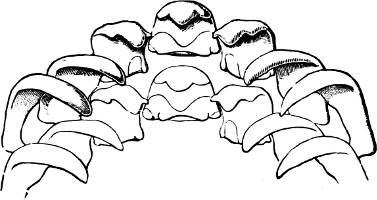
Fig. 127.—Two rows of the radula of Cypraea tigris L. × 30.
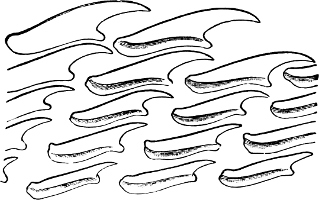
Fig. 128.—Portion of the radula of Ianthina communis Lam. × 40.
(d) Ptenoglossa.—This section consists of two families only, which certainly appear remarkably dissimilar in general habits and appearance, viz., the Ianthinidae and Scalariidae. In all probability their approximation is only provisional. The radula, which in Ianthina is very large, and in Scalaria very small, possesses an indefinite number of long hooked[225] teeth, of which the outermost are the largest. The central tooth, if present (it does not occur in Ianthina), is the smallest in the series, and thus recalls the arrangement in some of the carnivorous Pulmonata (p. 232). In Ianthina the radula is formed of two large divisions, with a gap between them down the middle.
The formula is ∞.1.∞ or ∞.O.∞ according as the central tooth in Scalaria is or is not reckoned to exist.
(e) Gymnoglossa.—In the absence of both jaw and radula it is not easy to classify the two families (Eulimidae and Pyramidellidae) which are grouped under this section. Fischer regards them as modified Ptenoglossa; one would think it more natural to approximate them to the Taenioglossa.
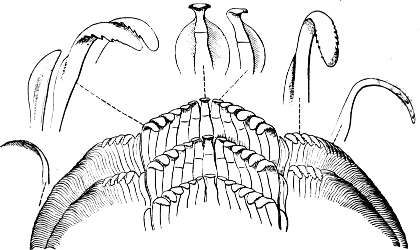
Fig. 129.—Portion of the radula of Margarita umbilicalis Brod., Labrador. × 75 and 300.
(f) Rhipidoglossa.—This section consists of seventeen families, the most important being the Helicinidae, Neritidae, Turbinidae, Trochidae, Haliotidae, Pleurotomariidae, and Fissurellidae. The radula is characterised by—
(1) The extraordinary development of the uncini, of which there are so many that they are always reckoned as indefinitely numerous. They are long, narrow, hooked, and often cusped at the top, and crowded together like the ribs of a fan, those at the extreme edge not being set straight in the row, but curving away backwards as they become smaller; in Solariella alone, where there are from five to ten, can they be counted.
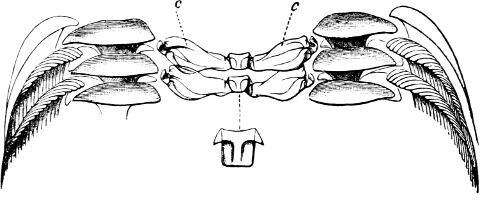
Fig. 130.—Portion of the radula of Nerita albicilla L., Andaman Is., with central tooth highly magnified: c, c, the capituliform tooth. × 40.
(2) The varying number of the laterals. The average number[226] of these is five on each side; in some cases (Livona) there are as many as nine, in some (Neritopsis) only three. The lateral next to the uncini (which is specially large in the Neritidae, and is then known as the capituliform tooth) is regarded by some authorities as the first uncinus, by others as the sole representative of the laterals, the teeth on the inner side of it being reckoned as multiplied central teeth. According to this latter view, Livona will have as many as seventeen central teeth. Taking five as the average number of ‘laterals,’ we shall have the following different ways of constituting the rhipidoglossate formula, the first being that to which preference is given, viz.:—
(1) ∞.5.1.5.∞, i.e. one central, five laterals, including the ‘last lateral’ tooth.
(2) (∞.1).4.1.4.(1.∞), regarding the ‘last lateral’ as first uncinus, but specialising it by a number.
(3) ∞.1.(4.1.4).1.∞, regarding the ‘last lateral’ as the only lateral.
In the Neritidae and the derived fresh-water genera (Neritina, Navicella) the first lateral, as well as the capituliform tooth, is very large, and in shape rather like the blade bone of a shoulder of mutton; the intervening laterals are very small. In Neritopsis (a degraded form) the central tooth and first lateral are entirely wanting. In the neritiform land-shells (Helicina, Proserpina) the first lateral is no larger than the others, while the capituliform tooth is enormous. Hydrocena is a very aberrant and apparently degraded form; the laterals between the first and the capituliform tooth are all wanting. In Haliotis, Scissurella, and Pleurotomaria the five laterals are of fairly[227] equal size; in Fissurella we again meet with a large capituliform tooth, with very small laterals.
(g) The Docoglossa are in direct contrast with the Rhipidoglossa in possessing few and strong teeth, instead of many and weak. There are only three families, Acmaeidae, Patellidae, and Lepetidae. In some of the Acmaeidae there are not more than two teeth in a row, while in no species are there more than twelve. The radula is, however, very long; there are as many as 180 rows in Patella vulgata. The teeth are thick, generally of a very deep red horn colour, rather opaque. The cartilage in which they are set is remarkably thick, and in some species whose teeth are very few a considerable portion of this cartilage is left quite bare.
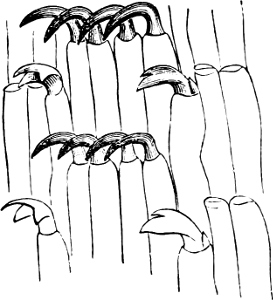
Fig. 131.—Portion of the radula of Patella cretacea Reeve, seen in half profile. × 40.
Although the teeth are so few, the arrangement is by no means simple. The special feature of the group is the multiplication of identical centrals. Of these there are two in Acmaea, and four, as a rule, in Patella. Thus in these two genera there is seldom an absolutely central tooth. Either laterals or marginals are liable to be lost, but there are never more than two of either in Acmaea, and never more than two laterals and three marginals in Patella. Thus the formula varies from 0.0.(1 + 0 + 1).0.0 in Pectinodonta, 2.2.(1 + 0 + 1).2.2 in Collisellina (both Acmaeidae), to 3.2.(1 + 0 + 1).2.3 in Patinella, and 3.1.(2 + 0 + 2).1.3 in Patella proper. In the Lepetidae there is an absolutely central tooth, which appears to be made up of the coalescence of several teeth, no laterals, and about two marginals; formula, 2.0.1.0.2.
Fig. 132.—Two rows of the radula of Pterotrachea mutica Les., Naples. × 60.
[228]
The radula of the Heteropoda is quite characteristic, and shows no sign of affinity with any other Prosobranchiate. The central tooth is large, broad, tricuspid, and denticulated on a broad base; the single lateral is strong, often bicuspid; the two marginals simple, long, falciform; formula, 2.1.1.1.2 (Fig. 132).
Fig. 133.—A, Portion of the radula of Chiton (Acanthopleura) spiniger] Sowb., Andamans, × 30; B, portion of the radula of Dentalium entalis L., Clyde, × 50.
Amphineura.—(a) Polyplacophora.—The radula of the Chitonidae is quite unique. It resembles that of the Docoglossa in being very long, and composed of thick and dark horn-coloured teeth. The number of teeth, however, is considerably greater, amounting almost invariably to seventeen in each row. There are three rather small central teeth, the two outer of these being similar; next comes a very large lateral (the major lateral), usually tricuspid, which is followed by two much smaller laterals, which are scarcely more than accessory plates; then a very large and arched marginal (the major uncinus), at the outer side of which are three accessory plates. Some consider there is only one central tooth, and count the two small teeth on each side of it as laterals.
Thus the formula is either (3 + 1).(2 + 1).3.(1 + 2).(1 + 3) or (3 + 1).(2 + 1 + 1).1.(1 + 1 + 2).(1 + 3).
(b) Aplacophora.—Of this rather obscure order, Chaetoderma has a single strong central tooth, Neomenia has no radula,[229] Proneomenia and Lepidomenia have about twenty falciform teeth, much larger at one end of the radula than the other; formula, 0.1.0.
Opisthobranchiata.—The radula of the Opisthobranchiata is exceedingly variable in shape, size, and number and character of teeth. Not only do allied families differ greatly from one another, but allied genera often possess radulae widely distinct in plan. Thus, among the Polyceridae, Goniodoris has no central tooth, one large lateral and one marginal (form. 1.1.0.1.1); Doridunculus the same, with five marginals (form. 5.1.0.1.5); Lamellidoris one each of median, laterals, and marginals (1.1.1.1.1); Idalia, Ancula, and Thecacera the same as Goniodoris; Crimora several each of laterals and marginals. Even species of the same genus may differ; thus the formula for Aeolis papillosa is 0.1.0, but for Ae. Landsbergi 1.1.1; for Philine aperta 1.0.1, but for Philine pruinosa 6.0.6.
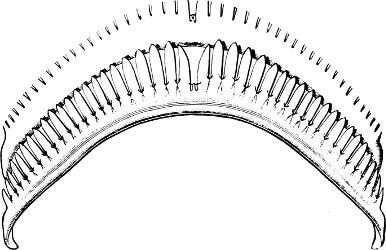
Fig. 134.—Two teeth from the radula of Aeolis papillosa L. × 55.
It must not be forgotten, however, that a simple repetition of the same tooth, whether lateral or marginal, does not necessarily constitute an important characteristic, nor does the presence or absence of a central tooth. In most of the cases mentioned above, the difference in the number of laterals and marginals is due to the multiplication of identical forms, while the central tooth, when present, is often a mere plate or narrow block without cusps, whose presence or absence makes little difference to the character of the radula as a whole.
There appear to be three well-marked types of radula among the Opisthobranchiata.
(a) Radula with a single strong central tooth, rows few.[230] This form is characteristic of the Aeolididae, Fionidae, Glaucidae, Dotoidae, Hermaeidae, Elysiidae (Fig. 135), and Limapontiidae. In the Aeolididae it is sometimes accompanied by a single lateral. The same type occurs in Oxynoe, and in Lobiger (= Lophocercus).
(b) Radula with the first lateral very strongly developed. This type may take the form of (1) a single lateral, no central or marginals, e.g. Onchidoris, Scaphander (Fig. 137, A), Philine (certain species), Ringicula, or (2) first lateral strongly developed, and repeated in succeeding laterals (2–6) on a smaller scale, e.g. Philine (certain species). A few marginals are sometimes added, e.g. in Polycera, Lamellidoris (where there is a degraded central tooth, Fig. 137, B), Idalia, and Ancula.
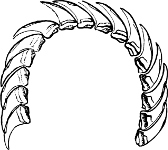
Fig. 135.—Radula of Elysia viridis Mont. × 40. Type (a).
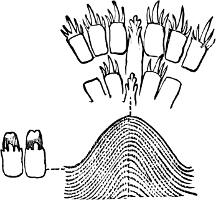
Fig. 136.—Portion of the radula of Gadinia peruviana Sowb., Chili. × 250. Type (c).
(c) Radula with an indefinite number of marginals, laterals (if present) merging into marginals, central tooth present or absent, inconspicuous, teeth all very small. This type of radula, among the Nudibranchiata, is characteristic of certain sub-genera of Doris (e.g. Chromodoris, Aphelodoris, Casella, Centrodoris), of Hypobranchiaea and Pleurophyllidia; among the Tectibranchiata, of Actaeon, many of the Bullidae, Aplustrum, the Aplysiidae, Pleurobranchus, Umbrella and Gadinia (Figs. 136 and 137, C).
In the Pteropoda there are two types of radula. The Gymnosomata, which are in the main carnivorous, possess a radula with a varying number (4–12) of sickle-shaped marginals, central tooth present or absent. In the Thecosomata, which feed on a vegetable diet, there are never more than three teeth, a central and a marginal on each side; teeth more or less cusped on a square base.
[231]
Pulmonata.—The radula of the Testacellidae, or carnivorous land Mollusca, is large, and consists of strong sickle-shaped teeth with very sharp points, arranged in rows with or without a central tooth, in such a way that the largest teeth are often on the outside, and the smallest on the inside of the row (as in Rhytida, Fig. 139). The number and size of the teeth vary. In Testacella and Glandina, they are numerous, consisting of from 30 to 70 in a row, with about 50 rows, the size throughout being fairly uniform. In Aerope they are exceedingly large, and only eight in a row, the outermost marginal being probably the largest single tooth in the whole of the Mollusca. The central tooth is always obscure, being, when present, simply a weaker form of the weakest lateral; in genera with only a few teeth in a row it is generally absent altogether.
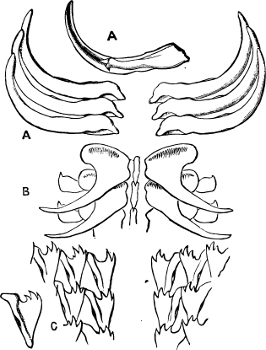
Fig. 137.—Portions of the radula of Opisthobranchiata, illustrating types (b) and (c); A, Scaphander lignarius L.; A´, one of the teeth seen from the other side, × 40; B, Lamellidoris bilamellata L., Torbay, × 60; C, Hydatina physis L., E. Indies, × 75.
The first family of jaw-bearing snails, the Selenitidae, is distinctly intermediate. The possession of a jaw relates it to the main body of Helicidae, but the jaw is not strong, while the teeth are still, with the exception of the central, thoroughly Testacellidan. The central tooth is quite rudimentary, but it is[232] something more than a mere weak reproduction of the marginals. There are no true laterals. The Limacidae show a further stage in the transition. Here the central tooth has a definite shape of its own, tricuspid on a broad base, which is more or less repeated in the first laterals; these, as they approach the marginals, gradually change in form, until the outer marginals are again thoroughly Testacellidan.[326] This is the general form of radula, varied more or less in different genera, which occurs in Nanina, Helicarion, Limax, Parmacella, and all the sub-genera of Zonites. It is certain that some, and probable that all of these genera will, on occasion, eat flesh, although their usual food appears to be vegetable. The jaw is more powerful than in the Selenitidae, but never so large or so strongly ribbed as in Helix proper.
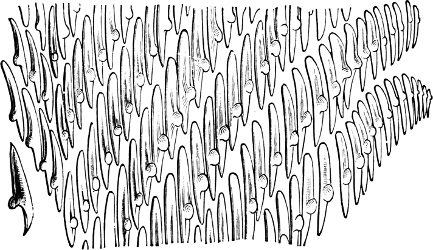
Fig. 138.—Portion of the radula of Glandina truncata Gmel. × 40.
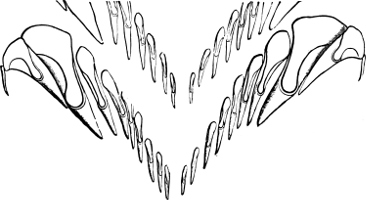
Fig. 139.—Portion of the radula of Rhytida Kraussii Pfr., S. Africa. × 25.
When we reach the Helicidae, we arrive at a type of radula[233] in which the aculeate form of tooth—so characteristic of the Agnatha—disappears even in the marginals, and is replaced by teeth with a more or less quadrate base; the laterals, which are always present, are intermediate in form between the central and the marginals, and insensibly pass into the latter. In size and number of cusps the first few laterals resemble the central tooth; in the extreme marginals the cusps often become irregular or evanescent. As a rule, the teeth are set squarely in the rows, with the exception of the extreme marginals, which tend to slope away on either side. In some Helicidae there is a slight approximation to the Zonitidae in the elongation of the first marginals.
The above is the type of radula occurring in the great family Helicidae, which includes not only Helix proper, with several thousand species, but also Arion, Bulimus, Ariolimax, and other genera. The jaw is almost always strongly transversely ribbed.
In the Orthalicidae (Fig. 140, C) the teeth of the radula, instead of being in straight rows, slope back at an angle of about 45 degrees from the central tooth. The central and laterals are very similar, with an obtuse cusp on rather a long stem; the marginals become bicuspid.
In the Bulimulidae, which include the important genera Placostylus, Amphidromus, Partula, Amphibulimus, and all the groups of South American Bulimulus, the jaw is very characteristic, being thin, arched, and denticulated at the edges, as if formed of numerous narrow folds overlapping one another. The radula is like that of the Helicidae, but the inner cusp of the laterals is usually lengthened and incurved. In Partula the separation between laterals and marginals is very strongly marked.
The remaining families of Pulmonata must be more briefly described. In the Cylindrellidae there are three distinct types of radula: (a) Central tooth a narrow plate, laterals all very curiously incurved with a blunt cusp, no marginals (Fig. 140, D); (b) radula long and narrow, central tooth as in (a), two laterals, and about eight small marginals; (c) much more helicidan in type, central and laterals obtusely unicuspid, marginals quite helicidan. Type (c) is restricted to Central America, types (a) and (b) are West Indian.
Pupidae: Radula long and narrow; teeth of the helicidan type, centrals and laterals tricuspid on a quadrate base, marginals very small, cusps irregular and evanescent. This type[234] includes Anostoma, Odontostomus, Buliminus, Vertigo, Strophia, Holospira, Clausilia, and Balea.
Stenogyridae, including Achatina, Stenogyra, and all its sub-genera: Central tooth small and narrow, laterals much larger, tricuspid, central cusp long, marginals similar, but smaller.
Achatinellidae: Two types occur; (a) teeth in very oblique rows, central, laterals, and marginals all of the same type, base narrow, head rather broad, with numerous small denticles (Achatinella proper, with Auriculella and Tornatellina, Fig. 140, E); (b) central tooth small and narrow, laterals bicuspid, marginals as in Helix (Amastra and Carelia).
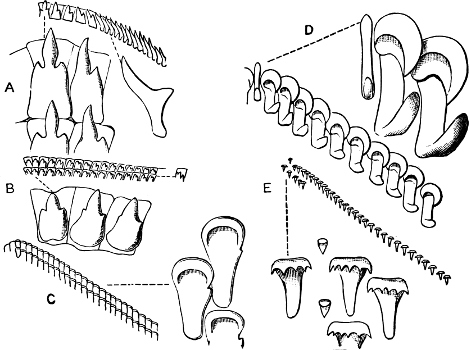
Fig. 140.—Portions of the radula of A, Hyalinia nitidula Drap., Yorkshire, with central tooth, first lateral, and a marginal very highly magnified; B, Helix pomatia L., Kent, showing central tooth, laterals, and one extreme marginal, the two former also highly magnified; C, Orthalicus undatus Brug., Trinidad, with three laterals highly magnified; D, Cylindrella rosea Pfr., Jamaica, central tooth and laterals, the same very highly magnified; E, Achatinella vulpina Fér., Oahu, central tooth (c) and laterals, the same highly magnified.
Succineidae: Central and laterals helicidan, bi- or tricuspid on a quadrate plate, marginals denticulate on a narrow base; jaw with an accessory oblong plate.
Janellidae: Central tooth very small, laterals and marginals like Achatinellidae (a).
Vaginulidae: Central, laterals, and marginals unicuspid throughout, on same plan.
Onchidiidae: Rows oblique at the centre, straight near the[235] edges; central strong, tricuspid; laterals and marginals very long, falciform, arched, unicuspid.
Auriculidae: Teeth very small; central narrow, tricuspid on rather a broad base; laterals and marginals obscurely tricuspid on a base like Succinea.
Limnaeidae: Jaw composed of one upper and two lateral pieces; central and lateral teeth resembling those of Helicidae; marginals much pectinated and serriform (Fig. 141, A). In Ancylus proper the teeth are of a very different type, base narrow, head rather blunt, with no sharp cusps, teeth similar throughout, except that the marginals become somewhat pectinated (Fig. 141, B); another type more resembles Limnaea.
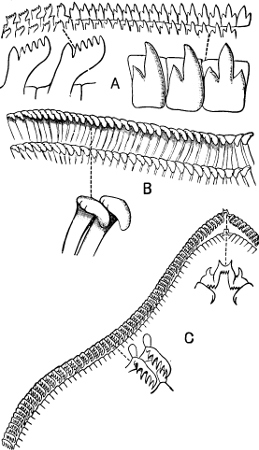
Fig. 141.—Portions of the radula of A, Limnaea stagnalis L., with the central tooth and two first laterals, and two of the marginals, very highly, magnified; B, Ancylus fluviatilis Müll., with two of the marginals very highly magnified; C, Physa fontinalis L., with central tooth and two of the marginals very highly magnified.
Physidae: Jaw simple, but with a fibrous growth at its upper edge, which may represent an accessory plate; radula with very oblique rows, central tooth denticulate, laterals and[236] marginals serriform, comb-like, with a wing-like appendage at the superior outer edge (Fig. 141, C).
Chilinidae: Central tooth small, cusped on an excavated triangular base, marginals five-cusped, with a projection as in Physa, laterals comb-like, serrations not deep.
Amphibolidae: Central tooth five-cusped on a broad base, central cusp very large; two laterals only, the first very small, thorn-like, the second like the central tooth, but three-cusped; laterals simple, sabre-shaped.
Scaphopoda.—In the single family (Dentaliidae) the radula is large, and quite unlike that of any other group. The central tooth is a simple broad plate; the single lateral is strong, arched, and slightly cusped; the marginal a very large quadrangular plate, quite simple; formula, 1.1.1.1.1 (Fig. 133, B).
Cephalopoda.—The radula of the Cephalopoda presents no special feature of interest. Perhaps the most remarkable fact about it is its singular uniformity of structure throughout a large number of genera. It is always very small, as compared with the size of the animal, most of the work being done by the powerful jaws, while the digestive powers of the stomach are very considerable.
The general type of structure is a central tooth, a very few laterals, and an occasional marginal or two; teeth of very uniform size and shape throughout. In the Dibranchiata, marginals are entirely absent, their place being always taken, in the Octopoda, by an accessory plate of varying shape and size. This plate is generally absent in the Decapoda. The central tooth is, in the Octopoda, very strong and characteristic; in Eledone and Octopus it is five-cusped, central cusp strong; in Argonauta unicuspid, in Tremoctopus tricuspid. The laterals are always three in number, the innermost lateral having a tendency to assume the form of the central. In Sepia the two inner laterals are exact reproductions of the central tooth; in Eledone, Sepiola, Loligo, and Sepia, the third lateral is falciform and much the largest.
Fig. 142.—Portion of the radula of Octopus tetracirrhus D. Ch., Naples, × 20.
In Nautilus, the only living representative of the Tetrabranchiata,[237] there are two sickle-shaped marginals on each side, each of which has a small accessory plate at the base. The two laterals and the central tooth are small, very similar to one another, unicuspid on a square base.
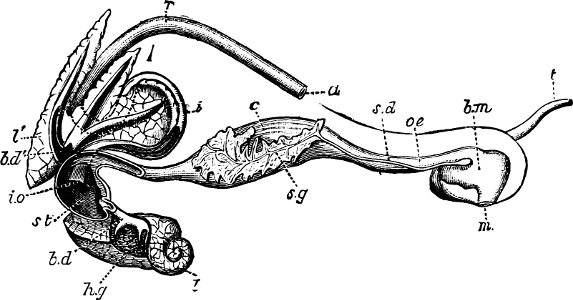
Fig. 143.—Alimentary canal of Helix aspersa L.: a, anus; b.d, b.d´, right and left biliary ducts; b.m, buccal mass; c, crop; h.g, hermaphrodite gland; i, intestine; i.o, opening of same from stomach (pyloric orifice); l, l´, right and left lobes of liver; m, mouth; oe, oesophagus; r, rectum; s.d, salivary duct; s.g, salivary gland; st, stomach; t, left tentacle. (After Howes and Marshall, slightly modified.)
Salivary glands are found in most Glossophora. They occur in one or two pairs on each side of the pharynx and oesophagus, the duct usually leading forwards and opening into the anterior part of the pharynx (see Figs. 143, 144). They are exceptionally large in the carnivorous Gasteropoda. In certain genera, e.g. Murex, Dolium, Cassis, Pleurobranchus, the secretions of these glands are found to contain a considerable proportion (sometimes as much as 4·25 per cent) of free sulphuric acid. This fact was first noticed by Troschel, who, while handling a Dolium galea at Messina, saw the creature spit a jet of saliva upon a marble slab, which immediately produced a brisk effervescence. A number of the genera thus provided bore through the shells of other Mollusca and of Echinoderms, to prey upon their soft tissues, and it is possible that the acid assists in the piercing of the shell by converting the hard carbonate of lime into sulphate of lime, which can easily be removed by the action of the radula.[327] In the majority of the Cephalopoda there are two pairs of salivary glands, one lying on each side of the mouth, the other on the middle of the oesophagus.
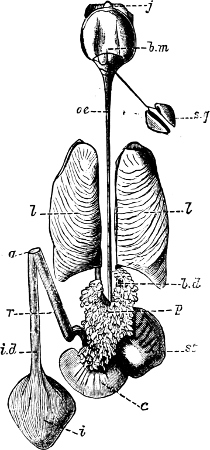
Fig. 144.—Alimentary canal, etc., of Sepia officinalis L.: a, anus; b.d, one of the biliary ducts; b.m, buccal mass; c, coecum; i, ink-sac; i.d, duct of same; j, jaws; l.l, lobes of the liver; oe, oesophagus; p, pancreatic coeca; r, rectum; s.g, salivary glands; st, stomach. (From a specimen in the British Museum.)
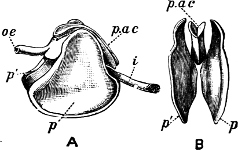
Fig. 145.—Gizzard of Scaphander lignarius L.: A, showing position with regard to oesophagus (oe) and intestine (i), the latter being full of comminuted fragments of food; p, left plate; p´, right plate; p.ac, accessory plate; B, the plates as seen from the front, with the enveloping membranes removed, lettering as in A. Natural size.
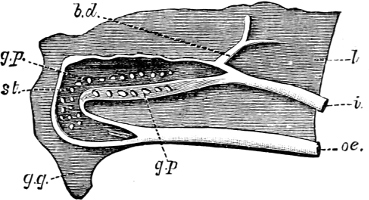
Fig. 146.—Section of the stomach of Melongena, showing the gastric plates (g.p, g.p,) for the trituration of food; b.d, biliary duct; g.g, genital gland; i, intestine; l, liver; oe, oesophagus; st, stomach. (After Vanstone.)
3. The Oesophagus.—That part of the alimentary canal which[238] lies between the pharynx and the stomach (in Pelecypoda between the mouth and stomach) is known as the oesophagus. Its exact limits are not easy to define, since in many cases the tube widens so gradually, while the muscular structure of its walls changes so slowly that it is difficult to say where oesophagus ends and stomach begins. As a rule, the oesophagus is fairly simple in structure, and consists of a straight and narrow tube. In the Pulmonata and Opisthobranchiata it often widens out into a ‘crop,’ which appears to serve the purpose of retaining a quantity of masticated food before it passes on to the stomach. In Octopus and Patella the crop takes the form of a lobular coecum. In the carnivorous Mollusca the oesophagus becomes complicated by the existence of a varying number of glands, by[239] the action of which digestion appears to begin in some cases before the food reaches the stomach proper.
4. The Stomach.—At the posterior end of the oesophagus lies the muscular pouch known as the stomach, in which the digestion of the food is principally performed. This organ may be, as in Limax, no more than a dilatation of the alimentary canal, or it may, as is usually the case, take the form of a well-marked bag or pocket. The two orifices of the stomach are not always situated at opposite ends; when the stomach itself is a simple enlargement of the wall of one side of the alimentary canal, the cardiac or entering orifice often becomes approximated to the orifice of exit (pyloric orifice).
The walls of the stomach itself are usually thickened and strengthened by constrictor muscles. In some Nudibranchs (Scyllaea, Bornella) they are lined on the inside with chitinous teeth. In Cyclostoma, and some Bithynia, Strombus, and Trochus there is a free chitinous stylet within the stomach.[328] In Melongena (Fig. 146) the posterior end of the oesophagus is provided with a number of hard plate-like ridges, while the stomach is lined with a double row of cuticular knobs, which are movable on their bases of attachment, and serve the purpose of triturating food.[329] Aplysia has several hard plates, set with knobs and spines, and similar organs occur in the Pteropoda. But the most formidable organ for the crushing of food is possessed by the Bullidae, and particularly by Scaphander (Fig. 145). Here there is a strong gizzard, consisting of several plates connected by powerful cartilages, which crush the shells, which are swallowed whole.
Into the stomach, or into the adjacent portions of the digestive tract, open the ducts which connect with the so-called liver. The functions of this important organ have not yet been thoroughly worked out. The liver is a lobe-shaped gland of a brown-gray or light red colour, which in the spirally-shelled families usually occupies the greater part of the spire. In the Cephalopoda, the two ducts of the liver are covered by appendages which are usually known as the pancreatic coeca; the biliary duct, instead of leading directly into the stomach, passes[240] into a very large coecum (see Fig. 144) or expansion of the same, which serves as a reservoir for the biliary secretions. At the point of connexion between the coecum and stomach is found a valve, which opens for the issue of the biliary products into the stomach, but closes against the entry of food into the coecum. In most Gasteropoda the liver consists of two distinct lobes, between which are embedded the stomach and part of the intestine. In many Nudibranchiata the liver becomes ‘diffused’ or broken up into a number of small diverticula or glands connecting with the stomach and intestine. The so-called cerata or dorsal lobes in the Aeolididae are in effect an external liver, the removal of which to the outside of the body gives the creature additional stomach-room.
The Hyaline Stylet.—In the great majority of bivalves the intestine is provided with a blind sac, or coecum of varying length. Within this is usually lodged a long cylindrical body known as the hyaline or crystalline stylet. In a well-developed Mytilus edulis it is over an inch in length, and in Mya arenaria between two and three inches. The bladder-like skin of the stylet, as well as its gelatinoid substance, are perfectly transparent. In the Unionidae there is no blind sac, and the stylet, when present, is in the intestine itself. It is said to be present or absent indifferently in certain species.
The actual function performed by the hyaline stylet is at present a matter of conjecture. Haseloff’s experiments on Mytilus edulis tend to confirm the suggestion of Möbius, that the structure represents a reserve of food material, not specially secreted, but a chemical modification of surplus food. He found that under natural conditions it was constantly present, but that specimens which were starved lost it in a few days, the more complete the starvation the more thorough being the loss; it reappeared when they were fed again. Schulze, on the other hand, believes that it serves, in combination with mucus secreted by the stomach, to protect the intestine against laceration by sharp particles introduced with the food. W. Clark found that in Pholas the stylet is connected with a light yellow corneous plate, and imagined therefore that it acts as a sort of spring to work the plate in order to comminute the food, the two together performing somewhat the function of a gizzard.[330]
[241]
5. and 6. The Intestine, Rectum, and Anus.—The intestine, the wider anal end of which is called the rectum, almost invariably makes a bend forward on leaving the stomach. This is the case in the Cephalopoda, Scaphopoda, and the great majority of Gasteropoda. The exceptions are the bilaterally symmetrical Amphineura, in which the anus is terminal, and many Opisthobranchiata, in which it is sometimes lateral (Fig. 68, p. 159), sometimes dorsal (Fig. 67). The intestine is usually short in carnivorous genera, but long and more or less convoluted in those which are phytophagous. In all cases where a branchial or pulmonary cavity exists, the anus is situated within it, and thus varies its position according to the position of the breathing organ. Thus in Helix it is far forward on the right side, in Testacella, Vaginula, and Onchidium almost terminal, in Patella at the back of the neck, slightly to the right side (Fig. 64, p. 157).
In the rhipidoglossate section of the Diotocardia (Trochus, Haliotis, etc.) the rectum passes through the ventricle of the heart, a fact which, taken in conjunction with others, is evidence of their relationship to the Pelecypoda.
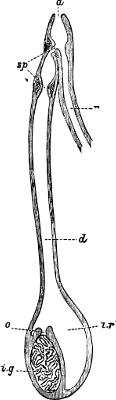
Fig. 147.—Ink-sac of Sepia, showing its relation to the rectum: a, anus; d, duct of sac; i.g, ink-gland; i.r, portion of the sac which serves as a reservoir for the ink; o, orifice of ink-gland; r, rectum; sp, double set of sphincter muscles controlling upper end of duct. (Modified from Girod.)
In nearly all Pelecypoda the intestine is very long and convoluted, being sometimes doubled forward over the mouth. Towards its terminal part it traverses the ventricle of the heart, except in Ostrea, Anomia, Teredo, and a few more. The anus is always at the posterior end of the animal, adjacent to and slightly above the adductor muscle.
Anal glands, which open into the rectum close to the anus, are present in some Prosobranchiata, e.g. Murex, Purpura. In the Cephalopoda the anal gland becomes of considerable size and importance, and is generally known as the ink-sac (Fig. 147);[242] it occurs in all known living genera, except Nautilus. The ink-sac consists of a large bag generally divided into two portions, in one of which the colouring matter is secreted, while the other acts as a reservoir for its storage. A long tube connects the bag with the end of the rectum, the mouth of the tube being controlled, in Sepia, by a double set of sphincter muscles.
The kidneys, nephridia,[331] renal or excretory organs, consist typically of two symmetrical glands, placed on the dorsal side of the body in close connexion with the pericardium. Each kidney opens on the one hand into the mantle cavity, close to the anus (see Fig. 64, p. 157), and on the other, into the pericardium. The venous blood returning from the body passes through the vascular walls of the kidneys, which are largely formed of cells containing uric acid. The blood thus parts with its impurities before it reaches the breathing organs.
The kidneys are paired in all cases where the branchiae are paired, and where the heart has two auricles, i.e. in the Amphineura, the Diotocardia (with the exception of the Neritidae), the Pelecypoda, and all Cephalopoda except Nautilus, which has four branchiae, four auricles, and four kidneys. In other Gasteropoda only one kidney survives, corresponding to the left kidney of Zygobranchiate Gasteropods.
Besides their use as excretory organs the kidneys, in certain groups of the Mollusca, stand in very close relation to the genital glands. In some of the Amphineura the generative products, instead of possessing a separate external orifice of their own, pass from the genital gland into the pericardium and so out through the kidneys (see Fig. 61 C, D, p. 154). In the Diotocardia it is the right kidney alone which serves, besides its excretory functions, as a duct for the emission of the generative products, the left kidney being at the same time greatly reduced in size. Thus in Patella the left nephridium is small, the right being much larger; both function as excretory organs, but the right serves as a mode of conveyance for the seminal products as well. In certain Pelecypoda (e.g. Yoldia, Avicula, Modiola, Pecten, Spondylus) the genital glands communicate directly,[243] and with a similar object, with the renal pouch on the same side of the body, but in the majority of cases the orifices are distinct.
The following memoirs will be found useful for further study of this portion of the subject:—
D. Barfurth, Ueber den Bau und die Thätigkeit der Gasteropodenleber: Arch. Mikr. Anat. xxii. (1883), pp. 473–524.
Th. Behme, Beiträge zur Anatomie und Entwickelungsgeschichte des Harnapparates der Lungenschnecken: Arch. Naturges. iv. (1889), pp. 1–28.
R. Bergh, Semper’s Reisen im Archipelago der Philippinen; Nudibranchiata: Theil ii. Band ii. (1870–78), Band iii. (1880–1892).
W. G. Binney, Terrestrial Air-breathing Mollusks of the United States: Bull. Mus. C. Z. Harv. iv. (1878), 450 pp.
„ On the Jaw and Lingual Membrane of North American Terrestrial Pulmonata: Proc. Ac. Nat. Sc. Philad. (1875), pp. 140–243.
J. T. Cunningham, The renal organs (Nephridia) of Patella: Quart. Journ. Micr. Sc. xxiii. (1883), pp. 369–375.
„ „ Note on the structure and relations of the kidney in Aplysia: Mitth. Zool. Stat. Neap. iv. (1883), pp. 420–428.
R. von Erlanger, On the paired Nephridia of Prosobranchs, etc.: Quart. Journ. Micr. Sc. xxxiii. (1892), pp. 587–623.
H. Fischer, Recherches sur la Morphologie du Foie des Gastéropodes: Bull. Scient. France Belg. xxiv. (1892), pp. 260–346.
C. Grobben, Morphologische Studien über den Harn- und Geschlechtsapparat, sowie die Leibeshöhle, der Cephalopoden: Arb. Zool. Inst. Wien, v. (1884), pp. 179–252.
„ Die Pericardialdrüse der Gasteropoden: ibid. ix. (1890), pp. 35–56.
B. Haller, Beiträge zur Kenntniss der Niere der Prosobranchier: Morph. Jahrb. xi. (1885), pp. 1–53.
A. Hancock, On the structure and homologies of the renal organ in the Nudibranchiate Mollusca: Trans. Linn. Soc. xxiv. (1864), pp. 511–530.
A. Köhler, Microchemische Untersuchung der Schneckenzungen: Zeits. Gesamm. Naturw. viii. (1856), pp. 106–112.
Ad. Oswald, Der Rüsselapparat der Prosobranchier: Jena. Zeits. Naturw. N.F. xxi. (1893), pp. 114–162.
R. Perrier, Recherches sur l’anatomie et l’histologie du rein des Gastéropodes prosobranches: Ann. Sc. Nat. Zool. (7), viii. (1889), pp. 61–315.
C. Semper, Reisen im Archipelago der Philippinen; Land Pulmonata: Theil ii. Band iii. (1870–77).
C. Troschel, Das Gebiss der Schnecken: Berlin, 1856–1892.
W. G. Vigelius, Ueber das Excretionssystem der Cephalopoden: Niederl. Arch. Zool. v. (1880), pp. 115–184.
[244]
The popular names of ‘shells,’ ‘shell-fish,’ and the like, as commonly applied to the Mollusca, the intrinsic beauty and grace of the shells themselves, resulting in the passion for their collection, their durability and ease of preservation, as compared with the non-testaceous portion,—all these considerations tend to unduly exalt the value of the shell as part of the organism as a whole, and to obscure the truth that the shell is by no means the most important of the organs.
At the same time it must not be forgotten that the old systems of classification, which were based almost entirely on indications drawn from the shell alone, have been strangely little disturbed by the new principles of arrangement, which depend mainly on structural points in the animal. This fact only tends to emphasise the truth that the shell and animal are in the closest possible connexion, and that the shell is a living part of the organism, and is equally sensitive to external influences.
A striking instance of the comparative valuelessness of the shell alone as a primary basis of classification is furnished by the large number of cases in which a limpet-shaped shell is assumed by genera widely removed from one another in cardinal points of organisation. This form of shell occurs in the common limpet (Patellidae), in Ancylus (Limnaeidae), Hemitoma (Fissurellidae), Cocculina (close to Trochidae), Umbrella and Siphonaria (Opisthobranchiata), while in many other cases the limpet form is nearly approached.
Roughly speaking, about three-quarters of the known Mollusca, recent and fossil, possess a univalve, and about one-fifth[245] a bivalve shell. In Pholas, and in some species of Thracia, there is a small accessory hinge plate; in the Polyplacophora, or Chitons, the shell consists of eight plates (see Fig. 2, p. 8), usually overlapping. A certain proportion of the Mollusca have no shell at all. In many of these cases the shell has been present in the larva, but is lost in the adult.
The shell may be
(1) External, as in the great majority of both univalves and bivalves.
(2) Partly external, partly internal; e.g. Homalonyx, Hemphillia, some of the Naticidae, Scutum, Acera, Aplustrum (Figs. 148 and 149).
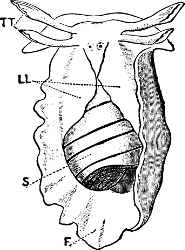
Fig. 148.—Aplustrum aplustre L. Mauritius, showing the partly internal shell (S); F, foot; LL, cephalic lappets; TT, double set of tentacles. (After Quoy and Gaimard.)

Fig. 149.—Sigaretus laevigatus Lam., showing shell partially immersed in the foot; F, anterior prolongation of the foot. (After Souleyet.)
(3) Internal; e.g. Philine, Gastropteron, Pleurobranchus, Aplysia, Limax, Arion, Hyalimax, Parmacella, Lamellaria, Cryptochiton, and, among bivalves, Chlamydoconcha.
(4) Absent; e.g. all Nudibranchiata and Aplacophora, many Cephalopoda, a few land Mollusca, e.g. all Onchidiidae, Philomycus, and Vaginula.
The Univalve Shell.—In univalve Mollusca the normal form of the shell is an elongated cone twisted into a spiral form round an axis, the spiral ascending to the left. Probably the original form of the shell was a simple cone, which covered the vital parts like a tent. As these parts tended to increase in size, their[246] position on the dorsal side of the animal caused them gradually to fall over, drawing the shell with them. The result of these two forces combined, the increasing size of the visceral hump, and its tendency to pull the shell over with it, probably resulted in the conversion of the conical into the spiral shell, which gradually came to envelop the whole animal. Where the visceral hump, instead of increasing in size, became flattened, the conical shape of the shell may have been modified into a simple elliptical plate (e.g. Limax), the nucleus representing the apex of the cone. In extreme cases even this plate dwindles to a few calcareous granules, or disappears altogether (Arion, Vaginula).
Varieties of the Spiral.—Almost every conceivable modification of the spiral occurs, from the type represented by Gena, Haliotis, Sigaretus, and Lamellaria, in which the spire is practically confined to the few apical whorls, with the body-whorl inordinately large in proportion, to a multispiral form like Terebra, with about twenty whorls, very gradually increasing in size.
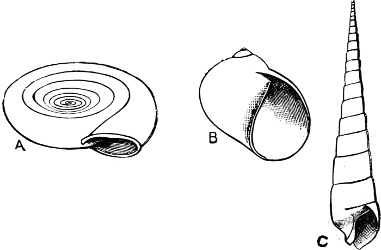
Fig. 150.—Examples of shells with A, a flattened spire (Polygyratia); B, a globose spire (Natica); C, a greatly produced spire (Terebra).
As a rule, the spire is more or less obliquely coiled round the axis, each whorl being partially covered, and therefore hidden by, its immediate successor, while the size of the whorls, and therefore the diameter of the spire as a whole, increases somewhat rapidly. The effect of this is to produce the elevated spire, the shell of six to ten whorls, and the wide aperture, of the normal type of mollusc, the whelk, snail, periwinkle, etc.
[247]
Sometimes, however, the coil of the whorls, instead of being oblique, tends to become horizontal to the axis, and thus we have another series of gradations of form, from the excessively produced spire of Terebra to the flattened disc of Planorbis, Polygyratia, Euomphalus, and Ammonites. The shell of many species of Conus practically belongs to the latter type, each whorl folding so closely over its predecessor that the spiral nature of the shell is not perceived until it is looked at at right angles to the spire.
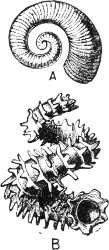
Fig. 151.—Examples of shells with disconnected whorls; A, Cyathopoma cornu Mf., Philippines; B, Cylindrella hystrix Wright, Cuba. (Both × 4.)
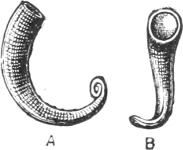
Fig. 152.—Example of a shell whose apical whorls alone are coiled, and the remainder produced in a regular curve. (Cyclosurus Mariei Morel., Mayotte.)
In some cases the regularly spiral form is kept, but the whorls are completely disconnected; e.g. some Scalaria, Spirula; among fossil Cephalopoda, Gyroceras, Crioceras, and Ancyloceras; and, among recent land Mollusca, Cylindrella hystrix and Cyathopoma cornu (Fig. 151). Sometimes only the last whorl becomes disconnected from the others, as in Rhiostoma (see Fig. 180, p. 266), Teinostoma, and in the fossil Ophidioceras and Macroscaphites. Sometimes, again, not more than one or two whorls at the apex are spirally coiled, and the rest of the shell is simply produced or coiled in an exceedingly irregular manner, e.g. Cyclosurus, Lituites, Orygoceras, Siliquaria (Fig. 153), Vermetus. In Coecum (Fig. 170, p. 260) the spiral part is entirely lost, and the shell becomes simply a cylinder. In a few cases the last whorl is coiled irregularly backwards, and is brought up to the apex, so that the animal in crawling must carry the shell with the spire downwards, as in[248] Anostoma (Fig. 154), Opisthostoma (Fig. 208, p. 309), Strophostoma, and Hypselostoma (Fig. 202 A, p. 302).
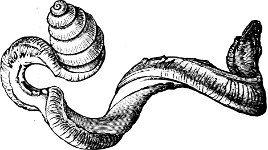
Fig. 153.—Siliquaria anguina Lam., showing scalariform coil of upper whorls and irregular extension of the lower.
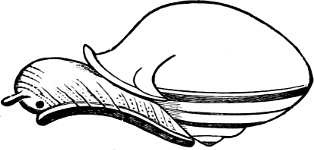
Fig. 154.—Anostoma globulosum Lam., Brazil. (After P. Fischer.)
Fig. 155.—Various forms of the internal plate in Capulidae: A, Calyptraea (Mitrularia) equestris Lam., E. Indies; B, Crucibulum scutellatum Gray, Panama; C, Ergaea plana Ad., and Reeve, Japan; D, Galerus chinensis L., Britain; E, Crepipatella dilatata Lam., Callao; F, Trochita maculata Quoy, N. Zealand; G, Crepidula fornicata Lam., N. America.
Some genera of the Capulidae, in which the shell is of a broadly conical form or with scarcely any spire, develop an internal plate or process which serves the purpose of keeping the animal within the shell, and does the work of a strong attachment muscle. In Mitrularia this process takes the form of a raised horse-shoe; in Crucibulum it is cup-shaped, with the edge free all round; in Galerus, Ergaea, Crepipatella, and Trochita we get a series of changes, in which the edge of the cup adheres to the interior of the shell, and then gradually flattens into a plate. In Crepidula proper this plate becomes a regular partition, covering a considerable portion of the interior (Fig. 155 G). Hipponyx secretes a thin calcareous plate on the ventral surface of the foot,[249] which intervenes like an operculum between the animal and the substance to which it adheres.
Sinistral, or Left-handed Shells.—The vast majority of univalve spiral shells are normally dextral, i.e. when held spire uppermost, with the aperture towards the observer, the aperture is to the right of the axis of the spire. If we imagine such a shell to be a spiral staircase, as we ascended it we should always have the axis of the spire to our left.
Sinistral or ‘reversed’ forms are not altogether uncommon, and may be grouped under four classes:—
(1) Cases in which the genus is normally sinistral; (2) cases in which the genus is normally dextral, but certain species are normally sinistral; (3) cases in which the shell is indifferently dextral or sinistral; (4) cases in which both genus and species are normally dextral, and a sinistral form is an abnormal monstrosity.
Fig. 156.—Fulgur perversum L., Florida. ½.
Fig. 157.—Illustration of the gradation of forms in Ampullaria between a dextral (A) and an ultra-dextral species (F).
In all cases of sinistral monstrosity, and all in which a sinistral and dextral form are interchangeable (sections 3 and 4 above), the position of the apertures of the internal organs appears to be relatively affected, i.e. the body is sinistral, as well as the shell. This has been proved to be the case in all specimens hitherto examined, and may therefore be assumed for the rest. The same uniformity, however, does not hold good in all cases for genera and species normally sinistral (sections 1 and 2). As a rule, the anal and genital apertures are, in these instances also, to the left, but not always. In Spirialis, Limacina, Meladomus, and Lanistes the shell is sinistral, but the animal is dextral. This apparent anomaly has been most ingeniously explained by Simroth, Von[250] Ihering, and Pelseneer. The shell, in all these cases, is not really sinistral, but ultra-dextral. Imagine the whorls of a dextral species capable of being flattened, as in a Planorbis, and continue the process, still pushing, as it were, the spire downwards until it occupies the place of the original umbilicus, becoming turned completely ‘inside out,’ and we have the whole explanation of these puzzling forms. The animal remains dextral, the shell has become sinistral. A convincing proof of the truth of this is furnished by the operculum. It is well known that the twist of the operculum varies with that of the shell; when the shell is dextral, the operculum is sinistral, with its nucleus near the columella, and vice versâ. In these ultra-dextral shells, however, where it is simply the method of the enrolment of the spire that comes in question, and not the formation of the whorls themselves, the operculum remains sinistral on the apparently sinistral shell.
The reverse case to this, when the shell is dextral but the orifices sinistral, is instanced by the two fresh-water genera Pompholyx (from N. America), and Choanomphalus (L. Baikal). A similar transition in the enrolment of the whorls may be confidently assumed to have taken place, and the shells are styled ultra-sinistral.
Yet another variation remains, in which the embryonic form is sinistral, but the adult shell dextral, the former remaining across the nucleus of the spire. This is the case with Odostomia, Eulimella, Turbonilla, and Mathilda, all belonging to the Prosobranchiata, with Actaeon, Tornatina, and Actaeonina among the Opisthobranchs, and Melampus alone among Pulmonates.
Monstrosities of the Shell.—Abnormal growths of the shell constantly occur, some of them being scarcely noticeable, except by a practised eye, others of a more serious nature, involving an entire change in the normal aspect of the creature. Scalariform monstrosities are occasionally met with, especially in Helix and Planorbis, when the whorls become unnaturally elevated, and sometimes quite disjoined from one another; carinated monstrosities develop a keel on a whorl usually smooth; acuminated monstrosities have the spire produced to an extreme length (Fig. 158); sinistral monstrosities (see above) have the spire reversed: dwarfs and giants, as in our own race, are occasionally noticed among a crowd of individuals.
[251]
More serious forms of monstrosity are those which occur in individual cases. Mr. S. P. Woodward once observed[332] a specimen of an adult Helix aspersa with a second, half-grown individual fixed to its spire, and partly embedded in the suture of the body whorl. The younger snail had died during its first hibernation, as was shown by the epiphragm remaining in the aperture, and its neighbour, not being able to get free of the incubus, partially enveloped it in the course of its growth. In the British Museum two Littorina littorea have become entangled in a somewhat similar way (Fig. 160 B), possibly as a result of embryonic fusion. Double apertures are not uncommon[333] in the more produced land-shells, such as Cylindrella and Clausilia (Fig. 160 A). In the Pickering collection was a Helix hortensis which had crawled into a nutshell when young, and, growing too large to escape, had to carry about this decidedly extra shell to the end of its days. A monstrosity of the cornucopia form, in which the whorls are uncoiled almost throughout, is of exceedingly rare occurrence (Fig. 161).
Fig. 158.—Monstrosities of Neptunea antiqua L., and Buccinum undatum L., with a greatly produced spire (from specimens in the Brit. Mus.).
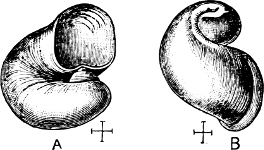
Fig. 159.—Monstrosities of Littorina rudis Mat, The Fleet, Weymouth. (After Sykes.)
Some decades ago ingenious Frenchmen amused themselves by creating artificial monstrosities. H. aspersa was taken from its shell, by carefully breaking it away, and then introduced into another shell of similar size (H. nemoralis, vermiculata, or[252] pisana). At the end of several days attachment to the columella took place, and then growth began, the new shell becoming soldered to the old, and the spiral part of the animal being protected by a thin calcareous envelope. A growth of from one to two whorls took place under these conditions. The individuals so treated were always sordid and lethargic, but they bred, and naturally produced a normal aspersa offspring.[334] In the British Museum there is a specimen of one of these artificial unions of a Helix with the shell of a Limnaea stagnalis.
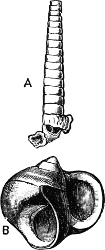
Fig. 160.—Monstrosities with two apertures: A, Cylindrella agnesiana C. B. Ad., Jamaica; B, Littorina littorea (from specimens in the British Museum).
Fig. 161.—Cornucopia-shaped monstrosity of Helix aspersa, from Ilfracombe. (British Museum.)
Composition of the Shell.—The shell is mainly composed of pure carbonate of lime, with a very slight proportion of phosphate of lime, and an organic base allied to chitin, known as conchiolin. The proportion of carbonate of lime is known to vary from about 99 p.c. in Strombus to about 89 p.c. in Turritella. Nearly 1 p.c. of phosphate of lime has been obtained from the shell of Helix nemoralis, and nearly 2 p.c. from that of Ostrea virginica. The conchiolin forms a sort of membranous framework for the shell; it soon disappears in dead specimens, leaving the shell much more brittle than it was when alive. Carbonate of magnesia has also been detected, to the extent of ·12 p.c. in Telescopium and ·48 p.c. in Neptunea antiqua. A trace of silica has also occasionally been found.
[253]
When the shell exhibits a crystalline formation, the carbonate of lime may take the form either of calcite or aragonite. The calcite crystals are rhombohedral, optically uniaxal, and cleave easily, while the aragonite cleave badly, belong to the rhombic system, and are harder and denser, and optically biaxal. Both classes of crystal may occur in the same shell.
Two main views have been held with regard to the formation and structure of the shell—(1) that of Bowerbank and Carpenter, that the shell is an organic formation, growing by interstitial deposit, in the same manner as the teeth and bones of the higher animals; (2) that of Réaumur, Eisig, and most modern writers, that the shell is of the nature of an excretion, deposited like a cuticle on the outside of the skin, being formed simply of a number of calcareous particles held together by a kind of ‘animal glue.’ Leydig’s view is that the shell of the Monotocardia is a secretion of the epithelium, but that in the Pulmonata it originates within the skin itself, and afterwards becomes free.[335]
According to Carpenter, when a fragment of any recent shell is decalcified by being placed in dilute acid, a definite animal basis remains, often so fine as to be no more than a membranous film, but sometimes consisting of an aggregation of ‘cells’ with perfectly definite forms. He accordingly divides all shell structure into cellular and membranous, according to the characteristics of the animal basis. Cellular structure is comparatively rare; it occurs most notably in Pinna, where the shell is composed of a vast multitude of tolerably regular hexagonal prisms (Fig. 162 B). Membranous structure comprises all forms of shell which do not present a cellular tissue. Carpenter held that the membrane itself was at one time a constituent part of the mantle of the mollusc, the carbonate of lime being secreted in minute ‘cells’ on its surface, and afterwards spreading over the subjacent membrane through the bursting of the cells.
The iridescence of nacreous shells is due to a peculiar lineation of their surface, which can be readily detected by a lens. According to Brewster, the iridescence is due to the alternation of layers of granular carbonate of lime and of a very thin organic membrane, the layers very slightly undulating. Carpenter,[254] on the other hand, holds that it depends upon the disposition of a single membranous layer in folds or plaits, which lie more or less obliquely to the general surface, so that their edges show as lines. The nacreous type of shell occurs largely among those Mollusca which, from other details in their organisation, are known to represent very ancient forms (e.g. Nucula, Avicula, Trigonia, Nautilus). It is also the least permanent, and thus in some strata we find that only casts of the nacreous shells remain, while those of different constitution are preserved entire.
Porcellanous shells (of which the great majority of Gasteropoda are instances) usually consist of three layers, each of which is composed of a number of adjacent plates, like cards on edge. The inclination of the plates in the different layers varies, but that of the plates in the inner and outer layer is frequently the same, thus if the plates are transverse in the middle stratum, they are longitudinal in the inner and outer strata, and, if longitudinal in the middle, they are transverse in the other two. Not uncommonly (Fig. 163 B) other layers occur. In bivalves the disposition and nature of the layers is much more varied.
Fig. 162.—A, Section of shell of Unio: a, periostracal layer; b, prismatic layer; c, nacreous layer. B, Horizontal section of shell of Pinna, showing the hexagonal prisms.
In Unio the periostracal or uppermost layer is very thin; beneath this is a prismatic layer of no great depth, while the whole remainder of the shell is nacreous (Fig. 162 A). Many bivalves show traces of tubular structure, while in the Veneridae the formation and character of the layers approaches closely to that of the Gasteropoda. Further details may be gathered from Carpenter’s researches.[336]
[255]
Formation of Shell.[337]—The mantle margin is the principal agent in the deposition of shell. It is true that if the shell be fractured at any point, the hole will be repaired, thus showing that every part of the mantle is furnished with shell-depositing cells, but such new deposits are devoid of colour and of periostracum, and no observation seems to have been made with regard to the layers of which they are composed. As a rule the mantle, except at its margin, only serves to thicken the innermost layer of shell.
It is probable that the carbonate of lime, of which the shell is mainly composed, is separated from the blood by the epithelial cells of the mantle margin, and takes the crystalline or granular form as it hardens on exposure after deposition. The three layers of a porcellanous shell are deposited successively, and the extreme edge of the mouth, when shell is forming, will contain only one layer, the outermost; a little further in, two layers appear, and further still, three. The pigment cells which colour the surface are situated at the front edge of the mantle margin.
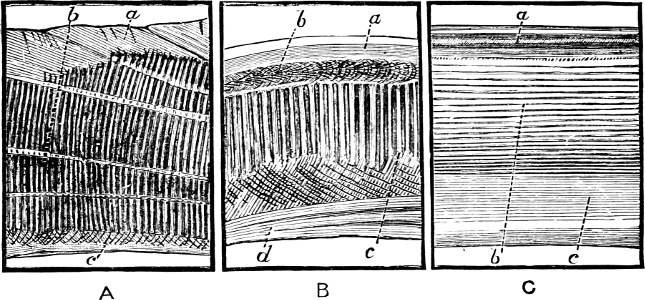
Fig. 163.—Sections of shells. A, Conus: a, outer layer; b, middle prismatic layer, with obliquely intersecting laminae above and below; c, inner layer. B, Oliva: a, outer layer; b, layer of crossed and curved laminae; c, prismatic layer, succeeded by layer of laminae at right angles to one another; d, inner layer. C, Cypraea: a, outer layer; b, middle layer; c, inner layer.
Shelly matter is deposited, and probably secreted, not only by the mantle, but also in some genera by the foot. This is certainly the case in Cymbium, Oliva, Ancillaria, Cassis, Distortio, and others, in several of which the foot is so large that the shell appears to be quite immersed in it.[338]
[256]
The deposition of shell is not continuous. Rest periods occur, during which the function is dormant; these periods are marked off on the edge of the shell, and are known as lines of growth. In some cases (Murex, Triton, Ranella), the rest period is marked by a decisive thickening of the lip, which persists on the surface of the shell as what is called a varix (see p. 263).
Fig. 164.—Murex tenuispina L., Ceylon. × ⅔.
Fig. 165.—Neritina longispina Récl., Mauritius. (Operculum removed.)
The various details of sculpture on the exterior surface of the shell, the striae, ribs, nodules, imbrications, spines, and other forms of ornamentation are all the product of similar and corresponding irregularities in the mantle margin, and have all been originally situated at the edge of the lip. Spines, e.g. those of Murex and Pteroceras, are first formed as a hollow thorn, cleft down its lower side, and are afterwards filled in with solid matter as the mantle edge withdraws. What purpose is served by the extreme elaboration of these spiny processes in some cases, can hardly be considered as satisfactorily ascertained. Possibly they are a form of sculptural development which is, in[257] the main, protective, and secures to its owners immunity from the attacks of predatory fishes.
‘Attached’ genera (e.g. Chama, Spondylus) when living on smooth surfaces have a flat shell, but when affixed to coral and other uneven surfaces they become very irregular in shape. The sculpture of the base on which they rest is often reproduced in these ‘attached’ shells, not only on the lower, but also on the upper valve, the growing edge of which rests on the uneven surface of the base. Oysters attached to the branches of the mangrove frequently display a central convex rib, modelled on the shape of the branch, from which the plaits of sculpture radiate, while specimens fixed to the smooth trunk have no such rib. Crepidula, a genus which is in the habit of attaching itself to other shells, varies in sculpture according to that of its host. Sometimes the fact may be detected that a specimen has lived on a ribbed shell when young, and on a smooth one when old, or vice versâ. A new genus was actually founded by Brown for a Capulus which had acquired ribs through adhesion to a Pecten. A specimen of Hinnites giganteus in the British Museum must at one period of its growth have adhered to a surface on which was a Serpula, the impression of which is plainly reproduced on the upper valve of the Hinnites.[339]
Fig. 166.—A specimen of Anomia ephippium L., Weymouth, taken upon Pecten maximus, the sculpture of which is reproduced on the upper valve of the Anomia, and even on a young Anomia attached to the larger specimen.
Growth of the Shell.—Nothing very definite is known with regard to the rate of growth of the shell in marine Mollusca. Under favourable conditions, however, certain species are known to increase very rapidly, especially if the food supply be abundant, and if there is no inconvenient crowding of individuals. Petit de la Saussaye mentions[340] the case of a ship which sailed from Marseilles for the west coast of Africa, after being fitted with an entirely new bottom. On arriving at its destination, the vessel spent 68 days in the Gambia River, and took 86 days on its homeward voyage. On being cleaned immediately on its[258] return to Marseilles, an Avicula 78 mm. and an Ostrea 95 mm. long (both being species peculiar to W. Africa) were taken from its keel. These specimens had therefore attained this growth in at most 154 days, for at the period of their first attachment they are known to be exceedingly minute. P. Fischer relates[341] that in 1862 a buoy, newly cleaned and painted, was placed in the basin at Arcachon. In less than a year after, it was found to be covered with thousands of very large Mytilus edulis, 100 mm. × 48 mm., the ordinary size on the adjoining banks being only about 50 to 60 × 30 mm.
Some observations have already been recorded (p. 40) on the growth of Helix aspersa. In the summer of 1858, which was very dry, especially in the south of France, the young Helices born that year were still very small in August. About the end of that month abundant rain came on, and in four or five days young H. variabilis, H. pisana, and H. aspersa, eating without cessation, as if to make up for lost time, grew more than a centimetre of shell. The lip of a young H. arbustorum has been observed to have grown, at the end of the first week in the season’s growth, 3 mm., at the end of the second week, 6·25 mm., the third, 11·5 mm., and the fourth 12·5 mm., with a finished lip.[342]
Careful observation has shown that in the growth of the shell of Helix aspersa the periostracum is first produced; it is covered with hyaline globules, 10–12 mm. in diameter, which persist even in the oldest shells. Calcareous matter is deposited on the internal face of the new periostracum, at some distance from the margin. It is secreted by a white zone or band of cells bounding the entire breadth of the mantle as applied to the peristome. Immediately behind the white zone are a series of pigment cells which not only give the shell its colour but complete the calcification of the shelly matter laid down by the white zone. When the animal has attained its full growth and the lip is finished off, the white band and the periostracum cells completely disappear, and only such cells persist as contribute to the internal thickening of the shell. Shell growth, in this species, is very rapid. If a portion of the pulmonary sac is laid bare, by removing a fragment of shell, at the end of 1½ or 2 hours there may be detected a delicate organic membrane covering the hole, and strewn with crystals of carbonate of lime. This thickens with[259] great rapidity, and soon fills up the hole with solid matter. For two consecutive months an animal, deprived of food, has been known to reproduce this membrane daily after its removal every morning.[343] Prof. Schiedt has found that oysters, if deprived of the right valve and exposed to the light, not only develop brown pigment over the whole exposed surface of mantle and branchiae, but actually succeed in part in reproducing the valve and hinge.[344]
Deposit of Additional Layers of Shell.—Mollusca possess the power of thickening the interior of the shell, by the deposit of successive layers. This is frequently done in self-defence against the attacks of boring Mollusca, sponges, and worms. Cases may often be noticed of Ostrea, Spondylus, and other sedentary molluscs, which, unable to escape the gradual assaults of their foes, have provided against them by the deposit of fresh shelly matter. A somewhat similar plan is adopted to provide against intrusion by way of the aperture. Pearls are, in many cases, the result of shell deposition upon the eggs or even the body of some intrusive parasite (Distoma, Filaria, etc.), and are, in some countries, artificially produced by the introduction of fragments of sand, metal, etc., into living Unio and Anodonta. Little joss images are made in India and China, the nacre on which is produced by thrusting them inside living Unionidae.
A specimen of Helix rosacea, in the British Museum, into whose shell a piece of grass somehow became introduced, has partitioned it off by the formation of a sort of shelly tunnel extending throughout its entire length (Fig. 167).
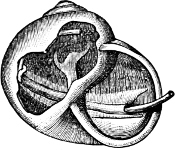
Fig. 167.—A specimen of Helix rosacea Müll., Cape of Good Hope, into which a piece of grass has by some means become introduced. The animal has protected itself by covering the grass with a shelly layer. (From a specimen in the British Museum.)
Absorption of Internal Portions.—Certain genera have the remarkable property of absorbing, when they become adult, the internal portions of the whorls and the greater part of the columellar axis. The effect of this is to make the shell, when the process is complete, no longer a spiral but a more or less produced cone, and it is found that in such cases the viscera of[260] the spire lose their spiral form, and take the shape of the cavity in which they lie. Amongst the genera in which this singular process takes place are Nerita,[345] Olivella, and Cypraea amongst marine forms, and nearly the whole of the Auriculidae[346] (Fig. 168). Conus reduces the internal subdivisions of the spire to extreme thinness. It is noticeable that these genera are all of considerable thickness of shell, and it is perhaps the result of the whole energy of the animal being directed to the formation of its external protection that the internal walls of the spire become atrophied and eventually disappear.

Fig. 168.—Auricula Judae Lam., showing the disappearance of the partitions of the whorls, which are represented by dotted lines. (After Fischer.)
Fig. 169.—A, Decollated (adult) form, and B, perfect (young) form of Cylindrella nobilior Ad., Jamaica; the dotted line shows where decollation takes place.
Fig. 170.—Development of Coecum: A, showing the gradual formation of septa; a, apex; ap, aperture; ss, first septum; s´s´, second septum. (After de Folin.) B, Adult form of C. eburneum Ad., Panama. × 8.
Decollation.—In certain genera, when the shell becomes adult, the animal ceases to occupy the upper whorls, which accordingly die and drop off, the orifice at the top having meanwhile been closed by a shelly deposit. Such shells are termed decollated. In some land genera decollation is the rule, e.g. in Cylindrella (Fig. 169), Eucalodium, and Rumina, as well as in many species of the brackish water genera, Truncatella, Cerithidea, and Quoyia. Stenogyra (Rumina) decollata, a common shell in the south of[261] Europe, has been noticed to bang its upper whorls violently against some hard substance, as if to get rid of them.
Fig. 171.—Four stages in the growth of Fissurella, showing how the spire gradually disappears and the marginal slit becomes an apical hole, A, B, C, highly magnified, D, natural size. (After Boutan.)
Fig. 172.—Three stages in the growth of Cypraea exanthema L. (From specimens taken at Panama.)
Special Points in the Growth of Certain Genera.—In the young of Coecum the apex is at first spiral, but as growth proceeds and the long tube begins to form, a septum is produced at the base of the apex, which soon drops off. Soon afterwards, a second septum forms a little farther down, and a second piece drops off, leaving the shell in the normal cylindrical form of the adult (Fig. 170). The development of Fissurella is of extreme interest. In an early stage it possesses a spiral shell, with a slit on the margin of the outer lip of the last whorl. As growth advances, shelly matter is deposited on both margins, which results in the slit becoming a hole and the spire a mere callosity, until at last they appear to coalesce in the apex of the adult shell (Fig. 171). The singular formations of Magilus and Rhizochilus have already been described (pp. 75, 76). Cypraea, in the young stage, is a thin spiral shell with a conspicuous apex. As growth proceeds,[262] the surface of the whorls, which are nearly enveloped by two large lobes of the mantle, becomes overlaid with new layers of shelly matter, until eventually the spire becomes embedded, and ultimately disappears from view (Fig. 172).
Patella, when young, has a nautiloid shell (see Fig. 45, p. 134), but it is a remarkable fact that we are entirely ignorant, in this commonest of molluscs, of the transition stages which convert the nautiloid into the familiar conical shell. The young shell of Pteroceras is deceptively unlike the adult, and is entirely devoid of the finger-like processes which are so characteristic of the genus (chap. xiv.).
Among the bivalve Mollusca, Anomia in a young stage is not to be distinguished from Ostrea. Soon a small sinus appears on the ventral margin, which gradually deepens and, as the shell grows round it, forms a hole for the byssus, eventually becoming fixed beneath the umbones (see Fig. 173). In Teredo the two valves of the shell proper, which is very small, become lodged in a long calcareous tube or cylinder, which is generally open at both ends (see chap. xvi.). In Aspergillum a somewhat similar cylinder is developed, but the valves are soldered to the tube, and form a part of it, the tube itself being furnished, at the anterior end, with a disc, which is perforated with holes like the rose of a watering-pot. In Clavagella the left valve alone becomes soldered to the tube, while the right valve is free within it (see chap. xvi.). Fistulana encloses the whole of its shell in a long tapering tube, which is not at any point adherent to the shell.
Fig. 173.—Development of the byssus or the plug-hole in Anomia. (After Morse.)
Terms employed to denote various Parts of the Univalve Shell.—The apex is the extreme top of the spire, and generally consists of the embryonic shell, which may often be recognised by its entire want of sculpture. When the embryonic shell happens to be large, the apex is often mammillated, e.g. in Fusus, Neptunea, and some Turbinella; in the Pyramidellidae it is sinistral.
The suture is the line of junction between any two successive[263] whorls. It may be deep, and even channelled, or very shallow, as in Fig. 150 B (p. 246).
The spire is the whole series of whorls except the last or body whorl. A whorl is a single revolution of the spiral cone round the axis. The spire may be subulate (as in Terebra, Fig. 150 C), turreted (Scalaria), depressed (Polygyratia, Fig. 150 A), conical (Trochus), globose (Ampullaria, Natica, Fig. 150 B), with almost all conceivable gradations between these types. The number of whorls is best counted by placing the shell mouth downwards, and reckoning one for every suture that occurs between the extreme anterior point of the shell and the apex.
Fig. 174.—Illustrating the technical terms applied to the various parts of a univalve shell.
The mouth or aperture may be (a) entire, as in Helix, Natica, Ampullaria, when its peristome or margin is not interrupted by any notch or canal, or (b) prolonged at its anterior and sometimes also at its posterior end into a canal. The anterior canal serves as a protection to the siphon,[347] the posterior canal is mainly anal in function, and corresponds, in part, to the hole of Fissurella, the slit in Pleurotoma and Emarginula, and the row of holes in Haliotis. The mouth presents every variety of shape, from the perfect circle in Cyclostoma and Trochus, to the narrow and prolonged slit in Conus and Oliva.
Fig. 175.—Anal slit in Pleurotoma.
The right margin of the mouth (the left, in sinistral shells)[264] is termed the outer lip or labrum, the left margin the inner lip, labium, or columella lip.[348] In young shells the outer lip is usually thin and unfinished, while in the adult it is generally thickened into a rib, or furnished with more or less prominent teeth, or given an inward or outward curve. In some genera, especially the Strombidae, the outer lip of the adult develops long finger-like processes, which sometimes attain an extraordinary size (chap. xiv.). As growth proceeds, these marginal teeth and ribs are either dissolved and disappear, or are permanently incorporated, in the shape of varices, with the framework of the shell. Some shells, e.g. Natica, Turritella, Actaeon, have a permanently unfinished outer lip, even in the adult stage. The columella lip varies in shape with the mouth as a whole; thus it may be straight, as in Conus, or excavated, as in Sigaretus, Struthiolaria, and Bulla. Frequently it is continued by part of the body whorl, as in Ficula, Dolium, and Fasciolaria.
Fig. 176.—Solarium perspectivum Lam., from the under side.
Fig. 177.—Section of Turbinella pyrum L., showing the plicae on the columella and the growth of successive whorls.
The folds or plaits on the columella, which are often characteristic of the genus or even family (e.g. Fasciolariidae, Mitridae, Turbinellidae) are not merely external, but continue down the[265] whole spire (see Fig. 177, which also shows how successive fresh growths have thickened the columella).
The whorls may be wound in a spiral, which is either hollow, as in Solarium, or quite compact, as in Oliva, Terebra, Cypraea, with every possible intermediate grade. This concavity, which varies in depth and width, is known as the umbilicus, and shells are accordingly spoken of as deeply (e.g. most Trochidae and Naticidae), narrowly (e.g. Lacuna, Littorina), or widely (e.g. Solarium) umbilicated. When the spiral is quite flat, as in Planorbis and some Helix, the umbilicus vanishes entirely. Shells in which the whorls are so compactly coiled on an ascending spiral that there is no umbilicus, are termed imperforate.
Fig. 178.—The slit in A, Hemitoma, B, Emarginula, C, Macroschisma, D, Craniopsis, E, Puncturella, F, Fissurella.
The Slit.—Many shells are furnished with a slit in the last whorl, which opens, in most cases, on the outer lip, and is sometimes of considerable depth, at others a mere notch. In the patelliform shells it is always in front of the apex. The function of the slit appears to be mainly anal, the excretory products being thus allowed to escape by a passage of their own, without soiling the clean water taken in by the branchiae. The posterior canal of some Gasteropoda probably performs a similar function. In the adult Fissurella the slit becomes an apical hole (see Fig. 178 F), in the allied genera it is either immediately in front of the spire (Puncturella), or half-way between the spire and the anterior margin (Rimula), or on the margin and well marked (Emarginula), or a mere indentation of the margin (Hemitoma). In Pleurotomaria it is exceptionally long, and is well marked in Bellerophon, Schismope, Scissurella, Murchisonia, and Pleurotoma (where it is sutural). In Haliotis and Polytremaria it is replaced by a series of holes, which are closed up as the animal grows past them. Some of these holes (at least in Haliotis) certainly[266] serve the purpose of admitting water to the branchiae, while others are anal. In Trochotoma there are only two holes, united by a narrow fissure.
The Tubed Land Operculates.—A group of the Cyclophoridae, which is restricted to Further India and the great Malay Islands, has developed a remarkable sutural tube on the exterior of the last whorl, near the aperture, A similar tube, but more obscure, exists in Alycaeus. Several stages in the development of this tube may be noticed, beginning with the elevation of part of the peristome into a simple irregular shelly plate, which is continued, first into a short, and then into a long tube, which becomes soldered to the shell; finally, the tube becomes free, and the anterior part of the last whorl is disconnected from the spire (Fig. 180 A-D).
Fig. 179.—The slit in A, Bellerophon, B, Pleurotomaria, C, Schismope, D, Polytremaria, E, Haliotis (not drawn to scale).
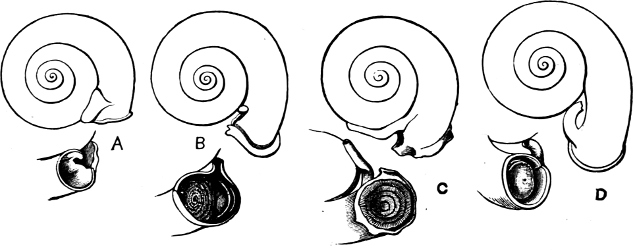
Fig. 180.—Development of the tube in the tube operculates: A, Pterocycus rupestris Bens.; B, Opisthoporus birostris Pfr.; C, Spiraculum travancoricum Bedd.; D, Rhiostoma Housei Pfr.
It is singular that the tube does not appear to be of any use to the animal, since its internal extremity, in the complete form, is closed, and does not communicate with the interior of the whorl. It may be presumed, however, that in origin the tube served as a means of conveying air to the animal when the operculum was closed. The holes in the peristome of Pupina, Cataulus, and Anostoma (Fig. 154) may be compared.
[267]
Fig. 181.—Eburna spirata Lam., E. Indies. F, foot; OP, operculum; P, penis; S, siphon; T, tentacles, with eyes at their base. (After Souleyet.)
The Operculum.—The operculum is a cuticular development of a group of cells situated on the dorsal side of the foot, exactly over the terminal point of the fibres of the columellar muscle. It is so situated that in crawling it is generally carried free of the shell, sometimes at the extreme upper end of the foot, more usually somewhat nearer to the shell (Fig. 181). In Pterocyclus it is pushed back into the umbilicus when the animal is in motion.
The operculum is present in nearly all land, fresh-water, and marine Prosobranchiata, absent in all Opisthobranchiata in the adult state, except Actaeon, and in all Pulmonata, except Amphibola. It has been lost in the following marine Prosobranchiata: many Cancellariidae and Conidae, Oliva (though present in Olivella and Ancilla), Harpidae, Marginellidae, Voluta proper (though present in V. musica), nearly all Mitridae, Cypraeidae, Doliidae, Ianthinidae; and, of land genera, in Proserpinidae. It is evident, therefore, that its presence or absence is of limited value in classification. In some species of Ampullaria and Natica it is horny, in others shelly. Dall found that in a number of specimens of Volutharpa ampullacea, 15 p.c. had opercula, 10 p.c. traces of the operculigenous area, but no operculum, the rest no trace of either. Monstrosities of Buccinum undatum sometimes occur, which have two, or in rare case three opercula.
As a rule, the operculum exactly fits the mouth of the shell. But in cases where the mouth is very large (e.g. Conus, Strombus, Concholepas, some Bullia), it only covers a very small portion and is quite inadequate as a protection (Fig. 62, p. 155). Again, when the shell has assumed a more or less limpet-shaped form, and habitually adheres to flat surfaces without much occasion for locomotion, the operculum becomes degraded and is probably on the way to being lost altogether. This is the case with Navicella (a modified Nerita, see Fig. 13, p. 17), Concholepas (a modified Purpura), Sigaretus (a modified Natica). Probably the more completely patelliform shells of Crepidula, Haliotis, Fissurella,[268] and Patella have reached the stage at which the operculum has been lost entirely. In Navicella, besides becoming degraded, the operculum has actually become partly internal, and apparently serves the purpose of separating the viscera from the upper part of the foot, something like the shelly plate in Crepidula. This explains why the operculum in this genus is polished on both sides.[349]
Some authors have imagined that the operculum is homologous (a) to the second valve in Pelecypoda, (b) to the byssus. It differs, however, morphologically from the former in the essential point of not being produced by the mantle, and from the latter in not being produced by a special gland.
Turbo (Sarmaticus) Turbo (Callopoma) Livona Ampullaria Natica
Fig. 182.—Various forms of opercula.
As regards shape and formation, the operculum has usually a more or less well-marked nucleus which may be central (e.g. Livona), sub-central (Ampullaria), lateral (Purpura), or terminal (Pyrula). As a rule, both the inner and outer surfaces are fairly flat, but in Torinia, Cyathopoma, and Pterocyclus the outer surface is elevated and conically spiral, in some Turbo (e.g. Sarmaticus) it is covered with raised tubercles resembling coral, while in others (e.g. Callopoma) it is scored with a deep trench. Aulopoma, a land genus peculiar to Ceylon, has a paucispiral operculum with hollow whorls, deceptively like a Planorbis; it fits over the aperture instead of into it. In Livona and most Trochidae the operculum is cartilaginous and multispiral. In Strombus it is narrow, curved, and often serrated like a leaf on one of the edges; in Conus it is narrowly oblong and rather featureless; in Littorina, paucispiral and always cartilaginous.[269] In many cases (e.g. Paludina) there is no true spiral form, but the striae are concentric to a nearly central nucleus, and thus give the appearance of a spiral. The evolution of the operculum in Navicella from Nerita has already been illustrated (p. 10). Neritopsis has a very remarkable operculum, the striated appendage of which locks behind the columella of the shell, like the tooth in the opercula of the Neritidae.
Pyrula Purpura Littorina Aulopoma × 3 Torinia × 2 Neritopsis Strombus Conus × 3/2
Fig. 183.—Various forms of opercula.
Terms employed to denote various parts of the Bivalve Shell.—The umbo, or beak, is the apex of the hollow cone, of which each valve may be regarded as consisting. This apex is usually more or less twisted: it is markedly spiral in Isocardia, Diceras, some Chama, and especially Requienia, while in Pecten, Lepton, and others the spiral is altogether absent. As a rule the umbones point forward, i.e. towards the anterior end of the shell. In Donax, Nucula, and Trigonia, however, they point backward. The umbones are generally more or less approximated, but in Arca they are widely separated.
An equilateral shell is one in which the umbones are more or less central with regard to its anterior and posterior portion, while in an inequilateral shell the umbones are much nearer one end than the other. On the other hand, equivalve and inequivalve are terms used to express the relation of the two valves to one another as a whole. Thus nearly all bivalve shells are more or less inequilateral, but a comparatively small proportion are inequivalve.
The dorsal margin is adjacent to, the ventral margin opposite to, the umbones. The anterior and posterior margins are respectively the front and hinder edges of the shell.
The muscles which serve to close the valves leave impressions on the inner surface of each valve. These, when both muscles are present, are known as the anterior and posterior adductor[270] impressions. The impression produced by the muscular edge of the mantle, which curves downwards and backwards from the anterior adductor impression, is known as the pallial line. In shells with only one muscle it is represented by an irregular row of small marks, or disappears altogether (Ostrea). The pallial sinus is produced by the muscles which retract the siphons, and is most marked in those genera in which the muscles are powerful and the siphons large (e.g. Tellina, Mya). It is entirely absent in genera possessing no retractile siphons.
Fig. 184.—Left valve of Venus gnidia L.: A, anterior, B, posterior, C, dorsal, D, ventral margin, AB, length, CD, breadth of shell.
a.m, anterior; p.m, posterior adductor muscle; p, pallial line; p.s, pallial sinus; l, ligament; lu, lunule; u, umbo; c, cardinal teeth; a.l, anterior lateral tooth; p.l, posterior lateral tooth.
Fig. 185.—Right valve of Lucina tigerina L.: A, anterior, B, posterior, C, dorsal, D, ventral margin; AB, length, CD, breadth of shell.
a.m, anterior; p.m, posterior adductor muscles; p, pallial line; l, ligament; u, umbo; c, cardinal teeth; a.l, p.l, anterior and posterior lateral tooth.
Right and Left Valve.—The simplest way of distinguishing the valves as right and left is to hold the shell in such a way that the siphons point towards the observer, and the mouth away from him; in this position the valve to the right is called the right valve, and the valve to the left the left valve. If, however, the animal is not present, it may be remembered that the ligament is nearly always behind the beaks, and that the beaks, as a rule, point forward, thus the right and left valves can generally be named by observation of the beaks and ligament. When the ligament is median to the valves (e.g. Ostrea, Pecten), and the beaks are not curved, the valves may be recognised by noting the fact that the impression of the adductor muscle (in these[271] cases always single) is nearer to the posterior than to the anterior side. In a similar way the pallial impression, which only forms a sinus on the posterior side, furnishes a guide to the valves of Donax, in which the beaks point backward, and of Tellina, in which the beaks are frequently central.
In the fixed inequivalves (e.g. Chama) it is sometimes the right, sometimes the left valve which is undermost, but the fixed valve, whether right or left, is always deep, and the free valve flat. Ostrea and Anomia are always fixed by the left valve.
The lunule is a well-marked area in front of and close to the umbones, usually more or less heart-shaped, and limited by a ridge. Generally it is shallow, but sometimes, as in Dosinia, Opis, and some Cardium, deeply impressed. A corresponding area behind the umbones, enclosing the ligament, is called the escutcheon (Fig. 186), but it seldom occurs.
The ligament is a more or less elastic band, which unites the two valves along a line adjacent to the umbones. As a rule, the greater part of the ligament is external to the shell, but it may be entirely internal. It is placed, normally, behind the umbones, but in a few cases, when the hinge line is very long (Arca, Pectunculus), it extends in front of them as well. The edges of the valves, when the ligament is mainly external, are more or less excavated for its reception. When internal it is generally contained in a groove or spoon-shaped pit, known as the fossette (compare Fig. 187).
Fig. 186.—Venus subrostrata Lam.: es, escutcheon; li, ligament; lu, lunule; u, u, umbones.
The ligament consists of two distinct parts, which may occur together or separately, the external, or ligament proper, and the internal, or cartilage. Only the external portion can be seen when the valves are closed. As a rule, the two portions are intimately connected with one another, the ligament folding over the cartilage, but in some cases, e.g. Mya, Mactra, where the cartilage is lodged within the hinge, they are completely disconnected (Fig. 187).
In Pecten the external ligament is very thin, and runs along the dorsal margin, while the internal ligament is large, solid, and situated in a shallow pit. In Perna, where the hinge is[272] toothless, the ligament is folded into a number of transverse ridges, which fit into corresponding grooves in the shell.
The ligament proper is inelastic and insoluble in caustic potash. The cartilage is very elastic, composed of parallel fibres, slightly iridescent, and soluble in caustic potash.
The operation of the ligament—using the word as including the whole ligamental process—is in opposition to that of the adductor muscles. When the latter close the valves, they compress the ligament, an action which its elasticity resists: thus its operation tends in part towards keeping the valves open. But when ligament and cartilage are both fully developed, they work in opposition to one another, the ligament, by its resistance to compression, preventing any straining of the adductor muscles when the valves are open, and the cartilage, for the same reason, preventing the ventral margins of the shell from closing too rapidly upon one another when the valves are being shut.
Fig. 187.—Hinge of A, right valve, and B, left valve of Mulinia edulis King; ca, cardinals; l.a, anterior laterals; l.p, posterior laterals; f, fossette; c, cartilage; l, ligament.
The Hinge.—The valves of Pelecypoda are generally articulated, below the umbones, by a hinge which is furnished, in the majority of cases, with interlocking teeth, small pits or depressions in each valve corresponding to the teeth in the other. The teeth are distinguished as cardinal, or those immediately below the umbo, and lateral, or those to either side of the cardinals, the latter being also distinguished as anterior and posterior laterals, according as they are before or behind the umbo (Fig. 184). In shells which are tolerably equilateral there is no difficulty in distinguishing between cardinal and lateral teeth. But when they are very inequilateral, the whole hinge may share in the inequality of growth, and an anterior lateral may be thrown backward and simulate a cardinal, or a cardinal may be thrown backward and simulate a posterior lateral (e.g. Cardita, Unio, Fig. 188). In many Chama the cardinals are pushed up into the umbo and become a mere ridge, while the[273] strong anterior lateral becomes nearly central and simulates a cardinal.
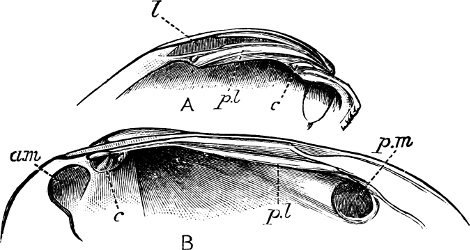
Fig. 188.—Hinges of A, Cardita semiorbiculata Brug., and B, Unio rectus Lam., showing how, in inequilateral shells, the lateral teeth tend to shift their position. a.m, anterior adductor, p.m, posterior adductor muscle; c, c, cardinal teeth; p.l, posterior lateral teeth; l, ligament.
Some bivalves, e.g. Anodonta, Ostrea, Pedum, many Mytilus, have no hinge teeth at all, in others the laterals are wanting (Psammobia, Diplodonta). In the Arcadae the hinge consists of a number of very similar denticles, which are often serrated like the teeth of a comb (Fig. 189).
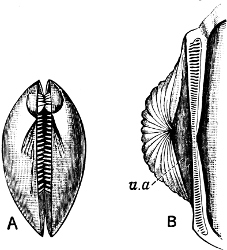
Fig. 189.—The hinge in Arcadae: A, Nacula Loringi Ang. × 4/3; B, Arca granosa L.; u.a, umbonal area.
Fig. 190.—A, Tridacna scapha Lam.; B, Cardium enode Sowb., showing the interlocking of the ventral margins.
Hinge-teeth are probably, in origin, derived from the crenulations or ribbings of the surface of the shell, the upper ends of which impinge upon the dorsal margin and mark it in a way which is quite recognisable when the shell is thin. Similar crenulations, resulting in interlocking of the valves, are not[274] uncommon upon the ventral margin in certain genera (Fig. 190). The mechanical effect of these continued riblets, when fitted together on the opposing valves, would be to prevent the valves sliding upon one another while closing, or after being closed. Thus there would be a probability of their surviving, even after the ribbing had disappeared from the surface of the shell, the increased strength given by the hinge compensating for, and making it possible to do without, the extra strength supplied by the ribs. It is therefore possible that the teeth of the Nuculidae and Arcadae, which have no distinction between cardinals and laterals, represent a very ancient type, from which have been evolved the various forms of hinge in which cardinals and laterals are distinguished. Even in some forms of Arcadae (comp. Pectunculus) we get a hint how the transverse teeth of the typical Arca may have become transformed into the longitudinal tooth of the normal lateral.[350]
The developed hinge-teeth, then, ensure the opening of the valves in one direction; they also secure their accurate closure upon one another in exactly the same plane. Exposed shells and gaping siphons matter little to animals which are protected by their burrowing propensities, but to those which live in material which can be easily penetrated by their foes, it must be of advantage to be able to buckle their armour absolutely tight. The edentulous hinge of Anodonta is a degeneration from a dentate type, which retains its teeth (in Unio, etc.) when subject to the jar of rapid streams, but tends to lose them in the stiller waters of canals, lakes, and ponds.
Other processes in the bivalve shell.—In Anatina each umbo is fissured and strengthened on the inside by a kind of umbonal plate which carries the ligament. Some forms of Liligna develop a strong internal umbonal rib, which serves as a buttress to strengthen the shell. In Pholas, the so-called falciform process serves as a point of attachment for the muscles of the foot and viscera. There is no ligament or hinge-teeth, the place of the latter being taken by the anterior adductor muscle, which is attached to the hinge-plate, the latter being reflected back into the shell.
In Septifer the anterior adductor muscle is carried on a sort of shelf or myophore, and in Cucullaea the posterior[275] adductor is partly raised on a similar and very prominent formation.
Length and breadth of bivalve shells is variously measured. Most authorities measure length, or ‘antero-posterior diameter,’ by a straight line drawn from the extreme anterior to the extreme posterior margin, and breadth by a similar line, drawn from the umbones to a point, not very clearly marked, on the opposite ventral margin (see Figs. 184 and 185). Others, less correctly, reverse these terms. Thickness is measured by the extreme distance of the opposite faces of the closed valves. As a rule, the length exceeds, and often greatly exceeds, the thickness, but in a few cases—e.g. the Cardissa section of Cardium—this is reversed.
The periostracum.—Nearly all shells are covered, at some period of their growth, by a periostracum,[351] or surface skin, which serves the purpose of protecting the shell against the destructive effects of the chemical action set up by water or air. It also, in some cases (see p. 258), acts as a kind of base upon which the shell is deposited. In old shells it is commonly worn away, especially at those parts which are likely to become abraded.
The form and composition of the periostracum varies greatly. Sometimes (e.g. Oliva) it is a mere transparent film, at others (Zonites) it is transparent, but stout and solid. It is corneous in Solenomya, covered with fine hairs in many Helicidae, in Conus, Velutina, and Cantharus it is thick, fibrous, and persistent; in Trichotropis and some Triton it is furnished with long bristles on a thick ground (Fig. 191). In fresh-water shells it is usually rather thick, in order to protect the shell from the erosive powers of certain kinds of water. In some cases (Mya, Anatina) the periostracum is continued over the siphons, so as to form a protection throughout their whole length.
Fig. 191.—Triton olearium L., Mediterranean, an example of a shell with a stout and hairy periostracum. × ½.
[276]
Erosion.—The fresh-water Mollusca generally, and marine mollusca in a few rare cases (Purpura, Littorina) are subject to erosion, or decay in the shell substance. In univalves erosion usually sets in near the apex (Fig. 192), where the life of the shell may be regarded as weakest, and in bivalves near the umbones. It is commonest in old shells, and rarely occurs in the very young. So long as the periostracum is present to protect the shell, erosion cannot set in, but when once it has been removed the shell is liable to the chemical changes set up in its substance by water. There is abundant evidence to show that erosion is caused by pollution of water. Out of many instances one must suffice. In a certain stream near Boston, U.S., numbers of Mollusca occurred, the shells of which were very perfect and free from disease. Some little way down stream an alkaline manufactory drained its refuse into the water. At and below this point for some distance every shell was more or less eroded, most of them seriously. Farther down, when the alkali refuse became diluted away, the shells retained their normal condition.[352]

Fig. 192.—Example of an eroded fresh-water shell (Melania confusa Dohrn, Ceylon).
A small percentage of lime in the water appears to produce erosion. The result of some experiments by G. W. Shrubsole, in the investigation of this point, may be tabulated as follows:[353]—
| Water from | Lime present per gall. |
Result | |
| River Dee, near Chester | 3·00 grs. | acted strongly | on shells |
| Wrexham | 4·00 grs. | „ „ | „ |
| River Dee, near Llanderyel | 0·53 grs. | „ „ | „ |
| Trent Canal | 8·33 grs. | no action | „ |
[277]
The Mollusca afford specially valuable evidence on problems of geographical distribution. This fact is largely due to their extreme susceptibility to any change in the conditions of life. Genera which are accustomed to live in a certain temperature and on certain food, cannot sustain life if the temperature falls or rises beyond certain limits, or if the required food be not forthcoming. There is therefore a marked contrast between the Mollusca of the tropics and of the temperate zones, while different regions in the same latitude, whether within or without the tropics, often show great diversity in their fauna. Every region is thus characterised by its Mollusca. The Mollusca, for instance, of Australia or of South Africa characterise those countries quite as much as do the kangaroo and the emu, the hartebeest and the ostrich; there is nothing like them anywhere else in the world. In the Greater Antilles the Mollusca stand out beyond all other forms of life as characteristic of the islands as a whole, and of each separate island in particular.
The geographical distribution of the land and fresh-water Mollusca must be considered quite apart from that of the marine Mollusca. The sea offers no such serious barriers to the spread of the latter as the land does to the spread of the former. If we were to journey to the Azores, and turn our attention to the land-snails, we should find them almost wholly peculiar, while amongst the sea-shells we should recognise many as occurring also on our southern coasts, and few that were different from those of the Mediterranean. The marine Mollusca of the Sandwich[278] Islands, in spite of the enormous intervening distance, are not very different from those of Natal, but the land Mollusca of the two countries are as widely different as is possible to imagine.
Land Mollusca are, as has been remarked, fettered to the soil. Quadrupeds, birds, fishes, and reptiles are provided with organs of motion which enable them to overpass barriers of various kinds. Even plants, although themselves incapable of motion, may be conveyed in every direction by means of seeds, which are either wafted by the wind or adhere to the skin of animals. But the Mollusca have no such regular means of transport, and are, in a large number of instances, limited to districts of a certain character of soil, or producing certain kinds of vegetation.
The localisation, both of genera and species, occurs all over the world. The genus Achatinella, which is peculiar to the Sandwich Islands, is found there in a profusion of species. It lives in the mountain valleys which radiate from the central ridge of each island, and each valley is characterised by its own peculiar set of species. The great carnivorous Glandina is restricted to Central America and the adjacent parts of the two continents, with one or two species in Southern Europe. Bulimus proper is restricted to South America; Achatina to Africa south of the Sahara; Tornatellina to the Pacific Islands; Cochlostyla to the Philippines; Cylindrella and Bulimulus are peculiar to the New World; Buliminus, Nanina, Scarabus, and Cassidula to the Old.
Extreme cases of this restriction of habitat sometimes occur. Thus Limnaea involuta is found only in a single small mountain tarn in Ireland; Clausilia scalaris along a narrow strip of limestone in Malta; Strophia nana is confined to a few square rods on an island that is itself a mere dot in the Caribbean Sea; the genus Camptonyx occurs only in the neighbourhood of Mt. Girnar, in Gujerat; and Lantzia in moss on the top of a mountain in Bourbon.
Attempts to colonise snails in strange localities have usually resulted in failure, especially when the attempt has involved serious changes of environment. The common Cochlicella acuta of our coasts resists all endeavours to establish it beyond a certain distance from the sea. Snails brought from the Riviera and placed under almost similar conditions of climate on our own southern coasts have lived for a while, but have very rarely taken[279] permanent root. Mr. H. W. Kew[354] has collected a good many of these attempts to acclimatise species, the general success of which seems to depend almost entirely on a restoration of the old conditions of life.
At the same time there are certain species which exhibit a curiously opposite tendency, and which seem capable of flourishing in almost any part of the world, and under the most varied surroundings. Our own common garden snail (Helix aspersa) is a striking instance of this adaptability to new conditions. It has been established, by art or by accident, in Nova Scotia, Maine, South Carolina, New Orleans, California, Mexico city, Cuba, Hayti, Cayenne, Brazil, Valparaiso, Cape Town, the Azores, St. Helena, Mauritius, Loyalty Islands, and Australia. The great Achatina fulica of East Africa has been established first in Mauritius, and from thence has been carried to the Seychelles and Calcutta. Helix lactea, a common Mediterranean species, has been carried to Teneriffe and Montevideo; Helix similaris, whose fatherland is Eastern Asia, has been transported to Mauritius, Bourbon, West Africa, West Indies, Brazil, and Australia; Ennea bicolor (Eastern Asia) to India, Bourbon, Mauritius, West Indies; Stenogyra decollata (Mediterranean basin) to South Carolina; S. Goodallii (West Indies) to British pineries; Helix Hortensis to New Jersey. Seven common English species (Limax gagates, Hyalinia cellaria, H. alliaria, Helix aspersa, H. pulchella, Pupa umbilicata) have become naturalised in St. Helena,[355] and as many as nineteen in Australia.[356]
Cases of artificial transport of this kind are readily detected; they follow the lines of trade. The snails themselves or their ova have been accidentally enclosed with plants or mould, or have adhered to packing-cases, or to hay and grass used in packing. Thus they constitute no disturbance to the general rule of the persistent localisation of species and genera, and there is little fear that the evidence which the geographical distribution of the Mollusca brings to bear upon the general problems of distribution will be confused by any intermixture of fauna naturally distinct.
Land Mollusca: Barriers to Dispersal.—The chief natural barriers to dispersal are extremes of temperature, the sea, mountain ranges, and deserts. Rivers, however large, seem of[280] little effect in checking dispersal. There is no appreciable difference between the land Mollusca north and south of the Ganges, or north and south of the Amazon. Living snails, or their ova, are no doubt transported from one bank to another on floating débris of various kinds. The barrier offered by the sea is obvious, and at first sight appears insurmountable; but the facts with regard to oceanic groups of islands like the Azores and Canaries (see p. 297) show that even a stretch of salt water many hundred miles in breadth may be ineffectual in preventing the dispersal of Mollusca.
Mountain ranges, provided they are too high to be scaled, and too long to be turned in flank, offer a far more effective barrier than the sea. Every thousand feet upward means a fall of so many degrees in the mean temperature, and a change, more or less marked, in the character of the vegetation. There is generally, too, a considerable difference in the nature of the climate on the two sides of a great mountain range, one side being often arid and cold, the other rainy and warm. The combined effect of these influences is, as a rule, decisive against the dispersal of Mollusca. Thus the Helices of California are almost entirely peculiar; one or two intruders from states farther east have succeeded in threading their way through the deep valleys into the Pacific provinces, but not a single genuine Californian species has been able to scale the heights of the Cascade Mountains. The land Mollusca of India are numbered by hundreds; not one penetrates north of the Himalayas. According to Mr. Nevill,[357] the change from the Indo-Malayan to the so-called European molluscan fauna at the northern watershed of the Kashmir valley is most abrupt and distinct; in two days’ march northward, every species is different. Ranges of inferior altitude, such as the Pyrenees, the Carpathians, or the Alleghanies, may be turned in flank as well as scaled, and we find no such marked contrast between the Mollusca on their opposite sides.
The most effective barrier of all, however, is a desert. Its scorching heat, combined with the absence of water and of vegetable life, check dispersal as nothing else can. The distribution of the Mollusca of the Palaearctic Region is an excellent instance of this. Their southern limit is the great desert which[281] stretches, with scarcely a break, from the west coast of Africa to the extreme east coast of Asia. The Mediterranean offers no effectual barrier; shells of southern Europe are found in profusion in Morocco, Tunis, and Egypt, while all through Siberia to the extreme of Kamschatka the same types, and even the same species, of Mollusca occur.
A detailed examination of the means, other than voluntary, by which Mollusca are transported from one place to another hardly comes within the scope of this work. Ocean currents, rivers, floods, cyclonic storms of wind, birds, and even beetles and frogs, play a part, more or less considerable, in carrying living Mollusca or their ova, either separately or in connexion with floating débris of every kind, to a distance from their native home. Accidental locomotion, of one or other of these kinds, combined with the well-known tenacity of life in many species (p. 37), may have contributed to enlarge the area of distribution in many cases, especially in the tropics, where the forces of nature are more vigorous than in our latitudes. The ease with which species are accidentally spread by man increases the probability of such cases occurring without the intervention of human agency, and numbers of instances may be collected of their actual occurrence.[358]
A point, however, which more concerns us here is to remark on the exceedingly wide distribution of the prevailing forms of fresh-water Mollusca. It might have been expected that the area of distribution in the fresh-water forms would be greatly restricted, since they cannot migrate across the land from one piece of water to another, and since the barriers between pond and pond, lake and lake, and one river system and another are, as far as they are concerned, all but insuperable. We might have expected, therefore, as Darwin and Wallace have remarked, to find a great multiplicity of species confined to very restricted areas, since the possibility of communication with the parent stock appears, in any given case, to be so exceedingly remote.
As is well known, the exact reverse occurs. The range, not merely of genera, but even of individual species, is astonishingly wide. This is especially the case with regard to the Pulmonata and Pelecypoda. The genera Limnaea, Planorbis, Physa, Ancylus, Unio, and Cyclas are world-wide. Out of about ten genera[282] of fresh-water Mollusca in New Zealand, one of the most isolated districts known, only one is peculiar. In South Africa and the Antilles no genus is peculiar. In the latter case, this fact is remarkable, when we consider that the same sub-region has at least ten peculiar genera of operculate land Mollusca alone.
To give a few instances of the distribution of particular species:—
Limnaea stagnalis L. occurs in the whole of Europe, and northern Asia to Amoorland, Turkestan, Afghanistan, North Persia, and Kashmir; Greenland, North America from the Atlantic to the Pacific, and from North Canada and British Columbia as far south as Texas. The distribution of L. peregra Müll., L. truncatula Müll., and L. palustris Müll, is almost equally wide.
Planorbis albus occurs in the whole of Europe, and northern Asia to Amoorland, Kamschatka, and Japan; Turkestan, the Altai-Baikal district, Alaska and Greenland, North Canada, and the whole of eastern North America.
The distribution of Anodonta anatina L., Cyclas cornea L., and Pisidium pusillum Gmel. is almost equally wide.
It is evident that the accidental means of transport mentioned above are insufficient to account for the facts as we find them; we are therefore compelled to seek for further explanation. Anything in the nature of a current furnishes a ready means of transport for Mollusca which have obtained a footing in the upper waters of a river, and there is no difficulty in imagining the gradual spread of species, through the agency of floods or otherwise, over a whole river system, when once established at any point upon it. The feeble clinging power of newly-hatched Limnaea has often been noticed as contributing to the chances of their range of distribution becoming extended. Fresh-water Mollusca, too, or their ova, are exceedingly likely, from their extreme abundance, to be transported by water-birds, which fly without alighting from one piece of water to another. Again, the isolation of one river system from another is, in many instances, by no means well marked or permanent, and a very slight alteration of level will frequently have the effect of diverting the supplies of one watershed into another. When we know what enormous oscillations in level have taken place over practically the whole surface of the globe, we can recognise the[283] probability that the whole river system of the earth has been mixed up and reconstructed again and again, with a very thorough blending of adjacent fauna.
It is possible that the very uniform conditions under which fresh-water Mollusca live may have something to do with the uniformity of their distribution and the comparative sameness in their development. There can scarcely be any question that the environments of fresh-water species are in themselves less varied and less liable to fluctuation than those of species whose home is the land. Water is very like water, all the world over; it may be running or motionless, warm or cold, clear or muddy, but the general tendency is for it to be free from extremes of any kind. Even if the surface water of a lake or river freezes, or becomes unusually hot, there is generally plenty of water at a lower stratum which maintains a less extreme temperature, and to which creatures can retire on the first symptoms of a change. From this two results will follow. Not only will the inhabitants of a piece of water not be inclined to vary much from the type, since their whole surroundings, food, etc., continue very much the same, but, if transported by any accident or cataclysm elsewhere, they will be exceedingly likely to arrive at a place which closely resembles their former home in all essentials. Thus the tendency for new types to be formed would be constantly checked, or rather would very seldom arise.
Mr. Belt, while recognising the importance of changes of level as affecting the distribution of fresh-water species, appears to regard the operations of such changes from a rather different point of view to that described above. “I think it probable,” he writes,[359] “that the variation of fresh-water species of animals and plants has been constantly checked by the want of continuity of lakes and rivers in time and space. In the great oscillation of the surface of the earth, of which geologists find so many proofs, every fresh-water area has again and again been destroyed.... Thus species of restricted range were always exposed to destruction, because their habitat was temporary and their retreat impossible, and only families of wide distribution could be preserved.”
The terrestrial surface of the globe has been divided, as indicating the facts of geographical distribution, into six regions—the[284] Palaearctic, Oriental, Australasian, Ethiopian, Nearctic, and Neotropical. To these is sometimes added a seventh, the Neantarctic, consisting of Chili and Patagonia (and certain islands of the south Atlantic); but since the Mollusca of Chili unmistakably form a part of the Neotropical fauna, it seems hardly worth while to recognise a separate region for those of the extreme south of South America, which have no peculiar characteristics.
In certain points the exact limits of these regions, as indicated by the Mollusca, will probably not correspond to those which are marked out by other zoological classes. Wallace’s line, for instance, does not exist, as far as the Mollusca are concerned.
These regions may be further subdivided into sub-regions, thus:—
| Regions | Sub-regions | |
| Palaearctic |  |
Septentrional |
| Mediterranean | ||
| Central Asiatic | ||
| Oriental |  |
Indo-Malay |
| Chinese | ||
| Australasian |  |
Papuan |
| Australian | ||
| Polynesian | ||
| Ethiopian |  |
Central African |
| South African | ||
| Malagasy | ||
| Nearctic |  |
American |
| Californian | ||
| Neotropical |  |
Antillean |
| Central American | ||
| Colombian | ||
| Brazilian | ||
| Chilian |
The southern boundary of this region is the northern limit of the African Sahara, the Mediterranean forming no break whatever in its continuity. In Asia this boundary is less well marked, but roughly corresponds to the southernmost of the vast ranges of mountains which border the great tablelands of central Asia. Across Africa the line of desert is well defined; but in the north-east, as the desert approaches more nearly to the sea, the African extent of the region is correspondingly narrowed until it becomes little more than a strip of coast land, scarcely widening even in Lower Egypt. On the Morocco coast, Palaearctic land forms penetrate as far south as Cape Nun.[360] At its eastern extremity the line becomes less well defined, but[285] probably proceeds along the snowy mountains west of Setchouan, the Pe-ling and Tan-sia-shan ranges, so as to include all the high ground of Thibet and of the upper waters of the Hoang-ho, and ultimately reaches its eastern limit at some point on the shores of the Sea of Japan.
The region thus includes all Europe, Africa north of the Sahara, with the Atlantic islands (the Azores, Canaries, etc.), North Arabia, Asiatic Turkey, the greater part of Persia, Afghanistan, Thibet, all Asiatic Russia, and a very large portion of the Chinese empire.
The principal characteristics of the region as a whole are:—
(1) The rich development of Helix, Arion, Limax, Buliminus, and Clausilia.
(2) The comparative absence of land operculates (see map, frontispiece).
(3) The uniform character of the fresh-water fauna.
It is in the southern portion of the region that Helix (in the sub-genera Macularia, Iberus, Pomatia, and Xerophila) and Buliminus (Zebrina, Chondrula, Ena) attain their maximum. In the north, Fruticicola is the characteristic group; in the mountainous districts of the south-east, Campylaea, with Clausilia. The Arionidae have their headquarters in the damp and warm regions of western Europe, but are rare in the south. They only approach the Mediterranean coast in Algeria, near Gibraltar, and in the region between the base of the Pyrenees and the Maritime Alps, and are very poor in species throughout Italy and Sardinia. They are absent from almost the whole of northern Africa, the Mediterranean islands (except Sardinia), the whole Balkan district, the Crimea, Caucasus, and western Asia.[361]
The uniformity of the fresh-water fauna is disturbed only in the extreme south. A few species of Melanopsis, with Neritina, occur in southern Spain and Austria, Galicia, and southern Russia, while a Melania or two (absent from Spain) penetrate the south-eastern parts of Europe as far as Germany. Cyrena begins to replace Cyclas in southern Russia and the Caucasus.
The Palaearctic region falls into three sub-regions:—
(1) The Northern or Septentrional Sub-region, i.e. the district[286] north of the line formed by the Pyrenees,[362] Alps, Carpathians, and which, passing to the northward of the Aralo-Caspian district, follows the great central mountain range of Asia until it reaches the Sea of Japan, perhaps somewhere in the neighbourhood of Vladivostok.
(2) The Mediterranean Sub-region, i.e. the countries bordering on the Mediterranean, the Black and Caspian Seas, with the Atlantic Islands.
(3) The Central Asiatic Sub-region, i.e. Turkestan, Afghanistan, Thibet, and probably the districts of Mongolia and Manchuria.[363]
(1) The Septentrional Sub-region has been divided by some writers into two provinces, the European and the Siberian. There seems, on the whole, but little occasion to separate off northern Asia, the characteristic of which is, as will be seen below, rather the gradual disappearance, as we proceed eastward, of European species and genera, than the development of any new and peculiar groups. The remarkable fauna of Lake Baikal stands apart, not only from European, but also from the Siberian types occurring in its immediate neighbourhood.
On the whole, the Septentrional Sub-region is poor in species except those which inhabit fresh water. This fact is probably due to the extreme vicissitudes of temperature which prevail, and it is interesting to notice that the number of land Mollusca appears to touch its lowest point in districts where the annual range of temperature is greatest. On the other hand, in the western portions of the region, where the climate is moist and temperature more equable, the Mollusca are considerably more abundant and varied.
The line which separates the Septentrional from the Mediterranean Sub-region must of necessity be very roughly drawn, and stragglers from the south will be found to make their way northward, and vice versâ, under favouring circumstances of temperature and geological formation. Jordan has noticed[364] that species[287] which in southern countries are not confined to any particular quality of soil are in more northern latitudes found only on limestone, which absorbs more heat than other formations. Conversely, the higher elevations of the Alps, Pyrenees, and even Carpathians are like islands in a sea, and support a thoroughly northern fauna, quite strange to that of the plains below. Thus Helix harpa Say, a completely boreal shell, which is at home in Canada, Sweden, Lapland, and the Amoor district, is found on the Riffel Alp, at a height of 6000 feet.[365] Vertigo arctica Wall., a species abundant in Lapland, North Siberia, Iceland, and Greenland, occurs on the high Alps of the Tyrol.
Circumpolar Species.—A certain number of species are common to the extreme north both of the Palaearctic and Nearctic regions, and are, in fact, circumpolar. The number of these species, however, is so small, not exceeding about 40 species (= 16 genera), that it seems hardly worth while creating a special sub-region for their reception, particularly as no genus is peculiar. At the same time the fact is instructive as illustrating the close connexion of the northern districts of the two regions, a connexion which was no doubt more intimate in recent geological times than it is now.
The circumpolar genera are as follows. The list decisively sets forth the superior hardiness of the fresh-water as compared with the land genera:—
| Valvata | 1 sp. |
| Bithynia | 1 „ |
| Vitrina | 1 „ |
| Hyalinia | 4 „ |
| Helix | 2 „ |
| Patula | 2 „ |
| Pupa | 3 „ |
| Cionella | 1 „ |
| Succinea | 1 „ |
| Limnaea | 7 „ |
| Planorbis | 5 „ |
| Aplecta | 1 „ |
| Physa | 1 „ |
| Anodonta | 1 „ |
| Unio | 1 „ |
| Pisidium | 1 „ |
Great Britain.—There are in all about 130 species—83 land, 46 fresh-water; Limnaea involuta (mountain tarn near Killarney) appears to be the only peculiar species. There are 11 Hyalinia, 5 Arion, and 25 Helix, the latter belonging principally to the sub-genera Xerophila, Tachea, Trichia, and Fruticicola. Three Testacella are probably not indigenous, but are now so well established as to reckon in the total. Of the four Clausilia two reach Ireland and one Scotland; two do not occur north of the Forth. There are only two land operculates, one of which (Cyclostoma elegans) occurs in Ireland but not in Scotland, while the other (Acicula lineata) reaches the southern counties[288] of Scotland. Several species, e.g. Helix pomatia, H. obvoluta, H. revelata, H. cartusiana, H. pisana, Buliminus montanus, are restricted to the more southern or western counties; Geomalacus maculosus is confined to a district in south-western Ireland.
The Pleistocene beds of East Anglia contain a number of species now extinct in these islands, whose occurrence appears to indicate a warmer climate than the present. Such are Helix ruderata, H. fruticum, H. incarnata, Clausilia pumila, Unio littoralis, Hydrobia marginata, and Corbicula fluminalis.
Scandinavian Peninsula.—From Norway 121 species in all are recorded, and 148 from Sweden. The milder climate of Norway allows many species to reach a considerably higher latitude than in Sweden, thus in Sweden Limax maximus only reaches 62°, but in Norway 66° 50´. Similarly Arion hortensis and Balea perversa only reach 63° and 61° respectively in Sweden, but in Norway are found as far north as 69° and 67° 50´. Clausilia is represented by 9 species in southern Norway; one of these is found north of the Arctic circle. There are 4 Pupa, 9 Vertigo, and 11 Hyalinia, but Helix dwindles to 14, 9 of which occur north of the Arctic circle. No land operculates are found; Cyclostoma elegans, however, occurs in Jutland and Zealand, which practically form a part of this district.
Iceland.—Eleven species, all Scandinavian, occur. These are Arion 2, Limax 1, Helix 2 (arbustorum L. and hortensis Müll., the latter being found only on the warmer southern coast), Limnaea 1, Planorbis 1, Pisidium 4.
France.—The northern, central, and eastern districts belong to this sub-region, while the southern and western, in which an entirely new element occurs and many northern forms disappear, belong to the Mediterranean. Thus, for instance, Helix pomatia L., H. incarnata Müll., H. fruticum Müll., H. cantiana Mont., H. strigella Drap., H. rufescens Penn., H. plebeia Drap., are not found in southern France. No detailed enumeration of species is at present possible, the efforts of a large number of the leading French authorities being directed to indiscriminate species-making rather than to the careful comparison of allied forms. Perhaps the principal difference between the Mollusca of northern France and those of our own islands is the occurrence of two species of Pomatias. In the more elevated districts of eastern France (the Vosges, Jura, western Alps), a certain number of[289] species occur which are confined to the high grounds of south central Europe. Among these are Helix holoserica Stud., H. personata Lam., H. bidens Chem., H. depilata Drap., H. cobresiana Alt., H. alpina Faure.
The Pleistocene deposits of the valley of the Somme tell the same tale as those of eastern England, containing as they do species and even genera whose northern range is now much more limited. The Eocene fossils from the Paris beds show most remarkable relationships to genera now existing in the West Indies and Central America. Others again indicate affinities with India. Thus we find Ceres, Megalomastoma, and Tudora by the side of Leptopoma, Faunus, and Paludomus.
Germany.—The Mollusca of the plains of northern Germany are few and not striking, and exhibit little difference from those of our own islands. In the mountainous districts of the south and south-east, a number of new forms occur, amongst which are 3 species of Daudebardia, a remarkable carnivorous form, with the general appearance of a Vitrina; 24 of Clausilia, many Pupa, several Buliminus, 3 of the Campylaea group of Helix, stragglers from the Italo-Dalmatian fauna, and 1 of Zonites proper. Our familiar Helix aspersa is entirely absent from Germany. There are only 4 land operculates—Pomatias 2, Acicula 1, Cyclostoma 1, all of which occur exclusively in the south. Bithynella and Vitrella, two minute forms of fresh-water operculates akin to Hydrobia, occur throughout the district.
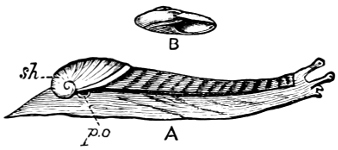
Fig. 193.—A, Daudebardia brevipes Fér.: sh, shell; p.o, pulmonary orifice. (After Pfeiffer.) B, shell of D. rufa Pfr., S. Germany.
Northern Russia and Siberia.—This vast tract extends from eastern Germany to the Amoor district. It is exceedingly poor in Mollusca, and is chiefly characterised by the gradual disappearance, as we proceed eastward, of European species. There are a few characteristic Siberian Mollusca, closely allied to European forms, and in the extreme east a new element is introduced in the appearance of types which indicate Chinese affinities. The whole district may be regarded as bounded to the south by a line drawn from Lemberg to Moscow, and thence to Perm; passing south of the Ural mountains, it includes the whole basins of the rivers Obi, Yenesei, and Lena, coinciding with[290] the vast mountain ranges which terminate to the north the table-land of central Asia, at the eastern extremity of which it dips sharply southwards, so as to include the Amoor basin and Corea.
All the larger Helices are wanting, and no land operculates occur. Helix arbustorum L., H. nemoralis Müll., H. lapicida L., H. aculeata Müll., and Hyalinia nitidula Drap., do not appear to occur east of the Baltic; Arion fuscus Müll., Helix strigella Drap., Buliminus obscurus Müll., Clausilia laminata Mont., C. bidentata Bttg., C. plicatula Drap., Viviparus fasciatus Müll., and Neritina fluviatilis L., do not pass the Urals.
In the Obi district (West Siberia) a further batch of European species find their easterly limit. Among these are Helix hispida L., Bithynia tentaculata L., Vivipara vivipara L., Pisidium amnicum Müll., and Unio tumidus Retz. A few distinctly Siberian species now appear, e.g. Ancylus sibiricus Gerst., Valvata sibirica Midd., and Vitrina rugulosa Koch.
The following are among the European species which reach eastern Siberia: Hyalinia nitida Müll., Succinea oblonga Drap., Planorbis vortex L., spirorbis L., marginatus Drap., rotundatus Poir., fontanus Light., Valvata piscinalis Müll., Bithynia ventricosa Leach, and Anodonta variabilis Drap. Here first occur such characteristic species as Physa sibirica West., P. aenigma West., Helix pauper Gld., H. Stuxbergi West., H. Nordenskiöldi West., Planorbis borealis Lov., Valvata aliena West., Cyclas nitida Cless., and C. levinodis West. In the Amoor district a decided Chinese element makes its appearance in a few hardy forms which have penetrated northward, e.g. Philomycus bilineatus Bens., and a few each of the Fruticicola (Chinese) and Acusta groups of Helix. Out of 53 species, however, enumerated from this district, as many as 33, belonging to 18 genera, occur also in Great Britain.
Lake Baikal.—The Mollusca of Lake Baikal exhibit distinct characteristics of their own, which seem to indicate the long-continued existence of the lake in its present condition. Several entirely peculiar genera occur, which are specialised forms of Hydrobia, e.g. Baikalia, Liobaikalia, Gerstfeldtia, Dybowskia, and Maackia; Benedictia alone extends to the basin of the Amoor. Choanomphalus, another peculiar and ultra-dextral (p. 250) genus belonging to the Limnaeidae, appears to be related to the West American Carinifex.
[291]
(2) The Mediterranean Sub-region is divided into four provinces: (a) The Mediterranean province proper; (b) the Pontic; (c) the Caucasian; and (d) the Atlantidean province.
(a) The Mediterranean province proper is best considered by further subdividing it, with Fischer and others, into separate districts, each of which has certain peculiar characteristics.
(i) The Hispano-Algerian district includes the greater part of the Iberian peninsula, the Balearic Islands, and northern Africa from Morocco to Tunis. The extreme western parts of these districts, including West Morocco, Portugal, Asturias, and south-west France, under the influence of the moist climate caused by the Atlantic, show some peculiar features which, in the view of some, are sufficient to justify their separation from the rest of the Hispano-Algerian portion. Among these is a marked development of the slugs, Testacella, Arion, and Geomalacus, the latter of which occurs even in south-western Ireland.
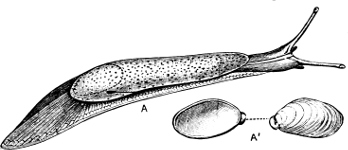
Fig. 194.—A, Parmacella Valenciensii W. and B. × ⅔. (After Moquin-Tandon.) A´, shell of the same, natural size.
Spain.—The principal features are the development of the Macularia, Iberus, and Gonostoma groups of Helix, and the occurrence of the remarkable slug Parmacella, which is found in many other parts of the sub-region, and extends eastward as far as Afghanistan. Clausilia has but few species, mostly in the north. There are four species of land operculates, one of which is referred to a genus (Tudora) now living only in the West Indies, but which occurs in the Eocene fossils of the Paris basin. In the south there are several species of Melanopsis and Neritina.
The States of Northern Africa have a thoroughly Mediterranean fauna, whose facies on the whole shows rather more affinity to Spain than to Sicily. The Helices of Morocco and Algeria belong to the same groups as those of southern Spain. Many are of a dead white colour, the better to resist the scorching effect of the sun. Ferussacia is abundant, Geomalacus and Parmacella[292] are represented by a single species each, and there is one Clausilia. According to Kobelt,[366] the original land connexion between southern Spain and Morocco must have been much more extensive than is usually assumed, and probably reached at least to the meridian of Oran and Cartagena. The Mollusca of Oran and Cartagena are, according to him, much more closely related than those of Oran and Tangier, or those of Cartagena and Gibraltar, but at Cartagena some species, which are characteristic of the Mediterranean coasts from Syria westward, disappear, are absent from the rest of Spain and from Morocco, but reappear on the south-western coasts of France. These species may possibly have pushed along that arm of the sea which, when the Straits of Gibraltar were closed as far as the latitude of Oran and Cartagena, united in comparatively recent times the Bay of Biscay with the Gulf of Lions.
The following genera, which do not occur in Spain, have probably spread into northern Africa as far as Algeria, via Sicily and Tunis, namely, Glandina (1 sp.), Daudebardia (1 sp.), Pomatias (2 sp.). Tunis shows strong traces of Sicilian influence, and Kobelt found a colony of snails, of Sicilian affinities, as far west as Tetuan.
The Sahara.—The Algerian Sahara contains, in many places, a sub-fossil Molluscan fauna which appears to show that the district has, in quite recent times, undergone a gradual desiccation. The species are mainly fresh-water, including Melania, Melanopsis, and Corbicula, with here and there valves of Cardium edule, and indicate, on the whole, an affinity with recent Egyptian, rather than North African species. It is probable that a vast series of étangs, or brackish-water lakes, once stretched along this region, and were ultimately connected with the sea somewhere between Tunis and Egypt.
Fig. 195.—Characteristic shells of S. France: A, Helix (Macularia) niciensis Fér.; B, Leucochroa candidissima Drap.
(ii) Southern France.—The southern portion of France bordering on the Mediterranean contains many species, especially of Helix, which do not occur in the centre and north. Amongst these are—
[293]
Several species of fresh-water Hydrobia (Bithynella) occur. The district, on the whole, unites certain characteristics derived from northern Italy with those of eastern Spain.
(iii) The Italo-Dalmatian district includes Italy and the neighbouring islands (Corsica, Sardinia, Sicily, Malta), and the regions at the head and north-eastern shores of the Adriatic (Carinthia, Carniola, Croatia, and Dalmatia), the line which separates these latter districts from the fauna of southern Austria, Bosnia, and Servia being very difficult to define.
Fig. 196.—Helix (Pomatia) aperta L., S. France, showing epiphragm.
Fig. 197.—Helix (Campylaea) zonata Stud., Piedmont.
Fig. 198.—Helix (Iberus) strigata Müll., Florence.
Italy, with the neighbouring islands, has a rich molluscan fauna. In the sub-Alpine districts of northern Italy the prominent Helix groups are Campylaea, Pomatia, and Anchistoma, which in the south are generally replaced by Iberus, which here attains its maximum development. Large Hyalinia are abundant in the north, and Pomatias and Clausilia are frequent all along the Apennines. Sicily has about 250 species, half of which are peculiar. Helices of the Iberus type abound, but Campylaea is reduced to two species. Many peculiar forms of Clausilia occur, especially a latticed type of great beauty. Ferussacia[294] and Pupa are well represented, and there are one each of Glandina and Daudebardia.
Dalmatia and the adjacent districts are chiefly remarkable for the rich development of Clausilia, which here attains its maximum (nearly 100 species). The Campylaea section of Helix is represented by its handsomest forms, many of which are studded with short hairs. Here too is the headquarters of Zonites proper, which stretches westward as far as Provence, and eastward to Asia Minor; and also of the single European Glandina, which has a similar eastward range, but spreads westward through Italy and Sicily to Algeria, not occurring in southern France. The land operculates are chiefly represented by Pomatias, and among the fresh-water operculates are a Melania and a Lithoglyphus, the latter having probably spread from the basin of the Danube.
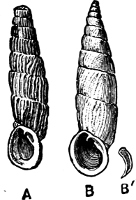
Fig. 199.—A, Clausilia crassicosta Ben., Sicily; B, Clausilia macarana Zieg., Dalmatia; B´, clausilium of same.
(iv) The Egypto-Syrian district extends along the south-eastern shores of the Mediterranean from Tripoli to North Syria, and eastward to the Euphrates valley. Lower Egypt alone belongs to this portion, the fauna of Upper Egypt being of an entirely tropical character, and belonging to the Ethiopian Region.
Lower Egypt.—The Mollusca of Lower Egypt stand in the unique position of belonging, half to the Palaearctic, and half to the Ethiopian Region. The land Mollusca are of a distinctly Mediterranean type, while the fresh-water, directly connected as they are by the great highway of the Nile with regions much farther south, contain a large admixture of thoroughly tropical genera (Ampullaria, Lanistes, Melania, Cleopatra, Corbicula, Cyrena, Iridina, Spatha, Mutela). The Helices, which are not numerous, are rather a mixture of circum-Mediterranean species than of a specially distinctive character. H. desertorum, however, belonging to the group Eremophila, is characteristic. There is a single Parmacella, but the physical features of the country are unfavourable to the occurrence of such genera as Clausilia, Pupa, Hyalinia, and the land operculates.
Syria.—The Mollusca, especially in the more mountainous[295] regions of the north, are much more varied and numerous than those of Egypt. Clausilia is again fairly plentiful, and the Helicidae are represented by some striking forms of the sections Levantina, Pomatia, and Nummulina. Leucochroa has several curious types with a constricted aperture, and the Agnatha are represented by Libania, a peculiar form of Daudebardia. A prominent feature is the occurrence of a number of large white Buliminus of the Petraeus section (Fig. 200). Land operculates appear to be absent, but Melanopsis and Neritina are abundant. The Dead Sea contains no Mollusca, but Lake Tiberias has a rich fauna, including the above-mentioned genera, with a Corbicula and several Unio.
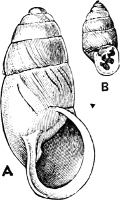
Fig. 200.—A, Buliminus (Petraeus) labrosus Oliv., Beyrout; B, Buliminus (Chondrula) septemdentatus Roth., Palestine.
Upper Mesopotamia appears to possess a mixture of Syrian and Caucasian forms, including a Parmacella. Lower Mesopotamia has an exceedingly poor land fauna, but is comparatively rich in fresh-water species, the growing eastern character of which is shown by the occurrence of several Corbicula and Pseudodon, and of a Neritina of a distinctly Indian type.
(b) The Pontic province extends from Western Austria to the Sea of Azof, and includes Austria, Hungary, Roumania, the Balkan peninsula (so far as it does not form part of the Mediterranean sub-region), the islands of the Greek Archipelago, southern Russia and the Crimea, and Asia Minor. It thus practically corresponds to the whole Danube basin, together with the lands bordering on the Black Sea, except at the extreme east, which belongs to the Caucasian sub-region. Fischer separates off Greece, Asia Minor (except the northern coast-line), and the intervening islands, with Crete and Cyprus, as constituting a portion (Hellado-Anatolic) of the Mediterranean sub-region proper. These districts, however, appear to possess scarcely sufficient individuality to warrant their separate consideration.
A prominent characteristic of the Pontic Mollusca is the great abundance of Clausilia and Buliminus. In the islands east and west of Greece Clausilia forms a large proportion of the fauna, each island, however small, possessing its own peculiar forms. The Helices belong principally to the groups Campylaea[296] (which is very abundant in Austro-Hungary), Pomatia (Greece and Asia Minor), and Anchistoma. Macularia is comparatively scarce, but is represented in Greece by one very large form (Codringtonii Gray). Zonites proper has its metropolis in this sub-region, and the Danube basin contains one or two species of Melania and Lithoglyphus. Buliminus is abundant throughout the sub-region, in the sub-genera Zebrina, Napaeus, Mastus, and Chondrula. Several striking forms of Zebrina (Ena) are peculiar to the Crimea.
(c) The Caucasian Province.—The limits of this province can hardly be exactly defined at present. It appears, however, to include the whole line of the Caucasus range, Armenia, and North Persia.
The land Mollusca are abundant and interesting. Among the carnivorous genera are four species of Daudebardia, a Glandina, and three peculiar forms of naked slug, Pseudomilax, Trigonochlamys, and Selenochlamys. There is a single Parmacella, the same species as the Mesopotamian, and a good many forms of Limax. Vitrina and Hyalinia are well represented, and the predominant groups of Helix are Euloto, Cartusiana, Xerophila, and Fruticocampylaea, the last being peculiar. Clausilia and Pupa are rich in species, together with Buliminus of the Chondrula type. One Clausilia of the Phaedusa section, together with a Macrochlamys (Transcaspian only), a Corbicula, and a Cyclotus, show marked traces of Asiatic affinity. There is one species each of Acicula and Cyclostoma, and one of Pomatias.
The Caspian Sea, like Lakes Baikal and Tanganyika, is distinguished by the possession of several remarkable and peculiar genera. The sea itself, the waters of which are brackish, is 80 feet below the level of the Black Sea, and is no doubt a relict of what formed, in earlier times, a very much larger expanse of water. Marine deposits containing fauna now characteristic of the Caspian, have been found as far north as the Samara bend of the Volga. It is probable, therefore, that in Post-pliocene times an arm of the Aralo-Caspian Sea penetrated northward up the present basin of the Volga to at least 54° N. The Kazan depression of the Volga (55° N.) also contains characteristic Caspian fossils.[367] According to Brusina,[368] the Caspian fauna,[297] as a whole, is closely related to the Tertiary fauna of southern Europe.
Twenty-six species of univalve Mollusca, the majority being modified forms of Hydrobia, have been described from the Caspian, namely, Micromelania (6), Caspia (7), Clessinia (3), Nematurella (3), Lithoglyphus (1), Planorbis (1), Zagrabica (1), Hydrobia (2), Neritina (2). The bivalves are mostly modified forms of Cardium (Didacna, Adacna, Monodacna), which also occur in estuaries along the north of the Black Sea. A form of Cardium edule itself occurs, and numberless varieties of the same species are found in a semi-fossil condition in the dry or half dry lake-beds, which are so abundant throughout the Aral district.
(d) The Atlantidean province consists of the four groups of islands, the Madeiran group, the Canaries, the Azores, and the Cape Verdes.
The Madeiran group contains between 140 and 150 species of Mollusca which may be regarded as indigenous, the great majority of which are peculiar. Only 11 species are common to Madeira and to the Azores, and about the same number, in spite of their much greater proximity, to Madeira and the Canaries. No less than 74 species, or almost exactly one-half, belong to Helix, and 9 to Patula. A considerable number of the Helices are not only specifically but generically peculiar, the genera bearing close relationship to those occurring in the Mediterranean region. As a rule they are small in size, but often of singular beauty of ornamentation. Various forms of Pupa are exceedingly abundant (28 sp.), as is also Ferussacia (12 sp.). There are also 3 Clausilia (which genus occurs on this group alone), and 3 Vitrina (a genus which occurs on all the groups). The land operculates are represented solely by 4 Craspedopoma, which is common to all the groups except the Cape Verdes.
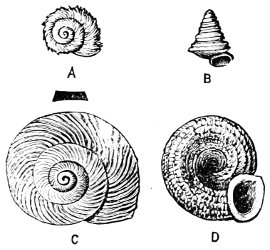
Fig. 201.—Characteristic land Mollusca from the Madeira group: A, Helix (Irus) laciniosa Lowe, Madeira; B, Helix (Hystricella) turricula Lowe, Porto Santo; C, Helix (Iberus) Wollastoni Lowe, Porto Santo; D, Helix (Coronaria) delphinuloides Lowe, Madeira.
The Canaries have about 160 species, only about a dozen of which are not peculiar. As many as 75 of these belong to[298] Helix (the sub-genera being very much the same as in the Madeiran group), and 11 to Patula. There is 1 species of Parmacella (which occurs in this group alone), and 6 of Vitrina, of considerable size. A remarkable slug (Plectrophorus) was described from Teneriffe by Férussac many years ago, but it has never been rediscovered, and is probably mythical, or wrongly assigned. Buliminus (Napaeus) has as many as 28 species, all but one being peculiar, and Ferussacia 7. Cyclostoma has two indigenous species, which, with one Hydrocena and one Craspedopoma, make up the operculate land fauna.
The Azores are comparatively poor in Mollusca, having only 52 species, nearly two-thirds of which are peculiar. Helix has 15 species, Patula 4, and Pupa 8. Ferussacia, so abundant in Madeira and the Canaries, is entirely absent, its place being taken by Napaeus (7 sp.), which is curiously absent from Madeira, but richly represented in the Canaries. There are 7 Vitrina, while the land operculates consist of one each of Craspedopoma and Hydrocena. A singular slug (Plutonia), with an ancyliform internal shell, is said to occur. The group was long believed to possess no fresh-water Mollusca, but two species (one each of Pisidium and Physa) have recently been discovered.
The Cape Verdes, owing to the extreme dryness of their climate, are poor in land Mollusca. There are 11 Helix, nearly all of which belong to the group Leptaxis, which is common to Madeira and the Canaries. Ferussacia is absent, Buliminus is represented by a single species, and there are no land operculates. Ethiopian influence, however, as might be expected from the situation of the group, is seen in the occurrence of an Ennea, a Melania, and an Isidora.
It will be noticed how little countenance the molluscan fauna of these island groups gives to any theory of an Atlantis, any theory which regards the islands as the remains of a western continent now sunk beneath the ocean. Had ‘Atlantis’ ever existed, we should have expected to find a considerable proportion of the Mollusca common to all the groups, and perhaps to Europe as well, and there would apparently be no reason why a genus which occurred in one group should not occur in all. As a fact, we find the species extremely localised throughout, and genera occur and fail to occur in a particular group without any obvious reason. All the evidence tends to show that the islands[299] are purely oceanic, and have been colonised from the western coasts of the Mediterranean, i.e. from the direction of the prevailing currents and winds.
(3) Central-Asiatic Sub-region.—The countries included in this vast sub-region are Turkestan, Songaria, Afghanistan, including the Pamirs, Western Thibet, and probably Mongolia. Kashmir belongs to the Indian fauna. At present the whole district is very imperfectly known; indeed, it is only at a few points that anything like a thorough investigation of the fauna has been made. It is therefore almost premature to pronounce any decided opinion upon the Mollusca, but all the evidence at present to hand tends to show that they belong to the Palaearctic and not to the Oriental system. This is especially the case with regard to the fresh-water Mollusca, many of which are specifically identical with those occurring in our own islands. A slight admixture of such widely distributed types as Corbicula and Melania occurs, but it is not sufficient to disturb the general European facies of the whole. It is possible that eventually the whole district may be regarded as a sub-region combining certain characteristics of the eastern portions of the Mediterranean basin with an extension of the septentrional element, due to higher elevation and more rigorous climate. The principal features in the land Mollusca appear to be the occurrence of a number of Buliminus of the Napaeus group, a few Parmacella (Afghanistan being the limit of the genus eastward), Clausilia, Pupa, Limax, and Helix, with several stray species of Macrochlamys.
This region includes all Asia to the south of the boundary of the Palaearctic region, that is to say, India, with Ceylon, Burmah, Siam, and the whole of the Malay Peninsula, China proper, with Hainan and Formosa, and Japan south of Yesso. It also includes the Andamans and Nicobars, and the whole of Malaysia, with the Philippines, as far eastward as, and including Celebes with the Xulla Is., and the string of islands south of the Banda Sea up to the Ké Is. The Moluccas, in their two groups, are intermediate between the Oriental and Australasian regions.
[300]
In this vast extent of land two distinct centres of influence are prominent—the Indian and the Chinese. Each is of marked individuality, but they differ in this essential point, that while the Chinese element is decidedly restricted in area, being, in fact, more or less confined to China itself and the adjacent islands, the Indian element, on the other hand, extends far beyond continental Asia, and embraces all the Malay islands to their farthest eastward extent, until it becomes overpowered by the Papuan and Australian fauna. Upper Burmah, with Siam, forms a sort of meeting-point of the two elements, which here intermingle in such a way that no very definite line of demarcation can be drawn between them.
Thus we have—
| Oriental Region | 1. Indo-Malay Sub-Region | (a) Indian Province |
| (b) Siamese Province | ||
| (c) Malay Province | ||
| (d) Philippine Province | ||
| 2. Chinese Sub-Region | (a) Chinese Province | |
| (b) Japanese Province |
The Indo-Malay fauna spreads eastward from its metropolis, but has practically no westward extension, or only such as may be traced on the eastern coasts of Africa and the off-lying islands. There appears to exist no other case in the world where the metropolis of a fauna is so plainly indicated, or where it lies, not near the centre, but at one of the ends of the whole area of distribution.
Comparing the two sub-regions, the Chinese is distinguished by the great predominance of Helix, while in the Indo-Malay sub-region Nanina and the related genera are in the ascendancy. In India itself there are only 6 genera of true Helicidae, poorly represented in point of numbers; in China there are at least three times this amount, most of them abundant in species. The Indo-Malay sub-region, on the other hand, is the metropolis of the Naninidae, which abound both in genera and species. In the Chinese sub-region Clausilia attains a development almost rivalling that of S.E. Europe, while in India there are scarcely a dozen species. A marked feature of the Indo-Malay sub-region is the singular group of tubed land operculates (Opisthoporus, Pterocyclus, etc.). In China the group is only represented by stragglers of Indian derivation, while the land operculate fauna,[301] as a whole, is distinctly inferior to the Indian. Another characteristic group of the Indo-Malay region is Amphidromus, with its gaudily painted and often sinistral shell; the genus is entirely absent from China proper and Japan, where its place is taken by various small forms of the Buliminus group. Fresh-water Mollusca, especially the bivalves and operculates, are far more abundant in the Chinese sub-region than in the Indo-Malay.
(1) The Indo-Malay Sub-region.—(a) The Indian Province proper includes the peninsula of Hindostan, together with Assam and Upper and Lower Burmah. To the east and extreme north-east, the boundaries of the province are ill-defined, and the fauna gradually assimilates with the Siamese on the one hand and the Chinese on the other. Roughly speaking, the line of demarcation follows the mountain ranges which separate Burmese from Chinese territory, but the debatable ground is of wide extent, and Yunnan, the first Chinese province over the border, has many species common with Upper Burmah.
The gigantic ranges of mountains which bound the sub-region to the north-west and north limit the extension of the Indian fauna in those directions in a most decisive manner. There is no quarter of the world, even in W. America, where a mountain chain has equal effect in barring back a fauna. In the north of Kashmir, where the great forests end, there is a most complete change of environment as the traveller gains the summit of the watershed; but Kashmir itself distinctly belongs to the Indian and not the Palaearctic system. The great desert to the south of the Punjab is equally effective as a barrier towards the west.
The Mollusca of India proper include a very large number of interesting and remarkable genera. India is the metropolis of the great family of the Naninidae, or snails with a caudal mucus-pore, which are here represented by no less than 14 genera and over 200 species. The genera Macrochlamys, Sitala, Kaliella, Ariophanta, Girasia, Austenia, and Durgella are at their maximum. Helix is scarcely represented, containing only about 30 inconspicuous species (leaving Ceylon out of account). Buliminus is abundant, especially in the north. The Stenogyridae are represented by Glessula, which is exceedingly abundant in India, but has only a few straggling representatives in the rest of the Oriental region. Among the Pupidae is the remarkable form[302] Boysia, with its twisted upturned mouth, while Lithotis is a peculiar form allied to Succinea, to which group also probably belongs Camptonyx, a limpet-like form with a very small spire, peculiar to the Kattiawar peninsula. Camptoceras, an extraordinarily elongated sinistral shell, with a loosely coiled spire, is peculiar to the N.W. Provinces.
Among the fresh-water pulmonates is an Ampullarina, a genus only known elsewhere from the Fiji Is. and E. Australia. Cremnoconchus is a form of Littorina, peculiar to the W. Ghâts, which has habituated itself to a terrestrial life on moist rocks many miles from the sea. The fresh-water operculates include the peculiar forms Mainwaringia, from the mouth of the Ganges (intermediate between Melania and Paludomus), Stomatodon, Larina, Fossarulus, Tricula, and others. The bivalves are neither numerous nor remarkable; Velorita, a genus of the Cyrenidae, is peculiar.
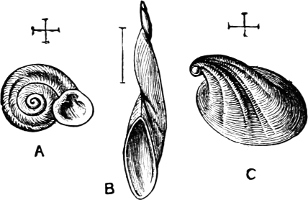
Fig. 202.—Characteristic Indian Mollusca: A, Hypselostoma tubiferum Blanf.; B, Camptoceras terebra Bens.; C, Camptonyx Theobaldi Bens.
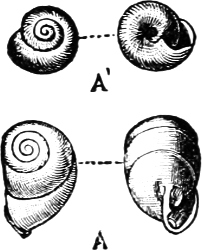
Fig. 203.—Streptaxis Perroteti Pfr., Nilghiri Hills: A, adult; A´, young form.
The land operculate fauna of India is singularly rich and varied. About 25 genera, and at least 190 species, occur. Here we find the metropolis of Cyclophorus among the larger forms, and of Diplommatina and Alycaeus among the smaller. A large proportion of the operculate genera are quite peculiar to the extreme south of India and Ceylon. The appearance of a few species of the European genus Pomatias is very remarkable.
The carnivorous genera are poorly represented. A few Ennea occur, while Streptaxis is practically confined to the extreme south and north-east.
[303]
Land and Fresh-water Mollusca of India proper
| Streptaxis | 9 |
| Ennea | 8 |
| Helicarion | 15 |
| Girasia | 14 |
| Austenia | 11 |
| Ibycus | 1 |
| Africarion | 2 |
| Durgella | 4 |
| Ariophanta | 15 |
| Xesta | 8 |
| Macrochlamys | 78 |
| Microcystis | 7 |
| Sitala | 20 |
| Kaliella | 35 |
| Hemiplecta | 15 |
| Sesara | 3 |
| Trochomorpha | 5 |
| Trochomorphoides | 1 |
| Parmacella (?) | 1 |
| Tebennophorus | 1 |
| Anadenus | 4 |
| Plectopylis | 11 |
| Plectotropis | 3 |
| Trachia | 12 |
| Thysanota | 1 |
| Camaena | 1 |
| Amphidromus | 2 |
| Boysia | 1 |
| Petraeus | 14 |
| Cerastus | 6 |
| Rachis | 5 |
| Cylindrus | 1 |
| Pupa | 15 |
| Hapalus | 4 |
| Clausilia | 10 |
| Subulina | 2 |
| Opeas | 6 |
| Glessula | 49 |
| Geostilbia | 3 |
| Succinea | 11 |
| Lithotis | 2 |
| Vaginula | 1 |
| Camptonyx | 1 |
| Coelostele | 1 |
| Carychium | 3 |
| Ancylus | 1 |
| Limnaea | 7 |
| Camptoceras | 3 |
| Planorbis | 10 |
| Ampullarina | 1 |
| Melania | 17 |
| Mainwaringia | 1 |
| Paludomus | 10 |
| Stomatodon | 1 |
| Larina | 1 |
| Cremnoconchus | 3 |
| Fairbankia | 2 |
| Tricula | 1 |
| Bithynia | 9 |
| Fossarulus | 1 |
| Stenothyra | 3 |
| Vivipara | 4 |
| Valvata | 1 |
| Ampullaria | 4 |
| Assiminea | 9 |
| Acmella | 2 |
| Pomatias | 4 |
| Diplommatina | 63 |
| Pupina | 1 |
| Streptaulus | 1 |
| Coptochilus | 3 |
| Alycaeus | 49 |
| Lagochilus | 1 |
| Cyclophorus | 12 |
| Scalaina | 1 |
| Micraulax | 2 |
| Jerdonia | 10 |
| Spiraculum | 4 |
| Otopoma | 1 |
| Cyclotopsis | 2 |
| Georissa | 1 |
| Modiola | 1 |
| Scaphula | 1 |
| Unio | 40 |
| Solenaia | 1 |
| Cyrena | 13 |
| Sphaerium | 1 |
| Pisidium | 5 |
| Velorita | 2 |
| Tanysiphon | 1 |
| Novaculina | 1 |
| Nausitora | 1 |
The Cingalese district, which almost approaches the character of a distinct province, presents several remarkable points of dissimilarity from the rest of India. It consists of the island of Ceylon, and of a portion of S. India whose exact limits have yet to be defined. It appears, however, that the Western or Malabar coast, with the hills parallel to it, is more akin to Ceylon than the Eastern or Coromandel coast. The Travancore, Malabar, and S. Canara districts, with the Palnai, Anamalai, and Nilghiri Hills, are markedly Cingalese, while there seems to be no distinct evidence of similar relationship on the part of the Madras or even the Cuddalore district.
Among the principal features of the Cingalese district is the occurrence of three peculiar genera of Helix, one (Acavus) large and finely coloured, another (Corilla) smaller, with a singularly toothed aperture. While the Corilla group shows relations with Plectopylis and other Burmese and Siamese sub-genera Acavus (Fig. 204) is totally distinct from any other Indian form, and shows signs of close relationship, in the great size of the embryonic shell, to the Helices of Madagascar (p. 335). In Ceylon the group is entirely isolated, and its occurrence, besides[304] decisively separating that island from India, Burmah, and Siam, forms a most interesting problem in the history of distribution. Eurystoma, with a single species (E. vittata Müll.), is also peculiar.
As usual when Helix gains ascendancy, the Naninidae retrogress. Durgella, Austenia, and Girasia are absent altogether, while Macrochlamys, Sitala, Kaliella, etc., are present in greatly diminished numbers. The sub-genus Beddomea is peculiar, a form directly related to Amphidromus (Siam and Malacca). The fresh-water operculate Philopotamis is peculiar, but for one species found in Sumatra; while Tanalia is quite peculiar. But the forms which, next to the Helices, most emphasise the separation of the Cingalese district are the land operculates. There are eleven genera or sub-genera of land operculates which do not occur in the rest of India proper. Two (Aulopoma and Cataulus) are quite peculiar, while the other nine are represented in Burmah, Siam, and the Malay islands, but not in India. On the other hand, Diplommatina and Alycaeus, so profusely abundant in India, have not yet been discovered in Ceylon. Among the slugs, Tennentia is a peculiar genus, whose nearest relation occurs in the Seychelles.
Fig. 204.—Helix (Acavus) Waltoni Reeve, Ceylon, showing embryonic shell (emb). × ⅔.
Genera and Subgenera occurring in the Cingalese District, but not in N. and Central India
The district consisting of Upper Burmah, Pegu, Tenasserim,[305] and Aracan, while essentially a part of the Indian province, contains several Siamese genera which are not found in India proper, as well as several which are at present peculiar. Amongst the former category are, of Helicidae, a single representative each of the genera Camaena (Siamese and Chinese) and Aegista (Chinese). Influence of the same kind is seen in the increased numbers of Plectopylis (14 sp.) and Plectotropis (5 sp.), of Clausilia (10 sp.) and Amphidromus (5 sp.), and of the large tubed operculates (11 sp. in all). Sesara and Sophina among the Naninidae are strange to India, while Hyalimax is common only to the Andamans, Nicobars, and Mascarene Is. Hypselostoma (Fig. 202, A) is a most remarkable genus of the Pupidae, reminding one of Anostoma of the New World. It is peculiar to the peninsula, but for one species in the Philippines. Among the Pupinidae, we have the peculiar Raphaulus and Hybocystis (Fig. 205), a very remarkable form, of which another species occurs at Perak. Two Helicina mark the most westward extension of the genus on the mainland. In the extreme north of Upper Burmah, Indian and Chinese forms intermingle.

Fig. 205.—Hybocystis gravida Bens. Young and adult.
The Burmese district, together with the Indian and Siamese provinces, is pre-eminently the home of a group of Mollusca, originally of marine origin, which have permanently habituated themselves to a brackish or fresh-water existence. They belong to widely different families, and even Orders. Besides Cremnoconchus mentioned above, we have, among the bivalves, Novaculina, a Solen living in fresh water in the Ganges, Irawadi, and Tenasserim estuaries; Scaphula, an Arca, one species of which occurs in the Ganges hundreds of miles above the tide-way (see Fig. 9, p. 14); and Martesia, a Pholas from the Irawadi Delta. Clea (which also occurs in Java and Sumatra) is probably an estuarine Cominella; a Tectura has earned the name fluminalis from its exclusive residence in the Irawadi R.; Iravadia is probably a Rissoina of similar habits, occurring from Ceylon round to Hong-Kong; Brotia is a Cerithium from an affluent of the River Salwin, and Canidia is a Nassa, occurring in the embouchures of rivers from India to Borneo. Nowhere else in the world is there such a collection—not exhausted by this list—of[306] marine forms caught in process of habituation to a fresh-water or even a land existence.
The Andaman and Nicobar Islands possess no peculiar features in their land Mollusca. They are closely related to the adjacent coasts of Lower Burmah. Amphidromus (2 sp.) occurs in the Andamans alone, and Clausilia (2 sp.) in the Nicobars alone, while Hyalimax occurs in both groups. A remarkable Helix (codonodes Fér.) from the Nicobars appears to find its nearest relations in the isolated group from Busuanga and Mindoro (p. 315). Land operculates are abundant, in the Nicobars actually outnumbering the pulmonates (28 to 22). Helicina and Omphalotropis, genera characteristic of small islands, are found on both groups.
(b) The Siamese Province includes the area occupied by the districts known as Siam, Laos, Cambodia, Cochin China, Annam, and Tonquin. Along the whole of its northern frontier, the zoological is no more than a political boundary, while on the east the mountain ranges which part Siam from Pegu and Tenasserim are not of sufficient height to offer any effective barrier to distribution. The province is accordingly qualified to a considerable extent by Indian and Chinese elements.
Streptaxis is, but for three Ennea, the sole representative of the carnivorous genera, and attains its maximum in the Old World. Partly owing to Chinese influence, the Helicidae, with 11 genera and 46 species, begin to regain their position as compared with the Naninidae (12 genera, 54 species). Of the Helicidae, Acusta and Hadra appear now for the first time, and, with Plectotropis, Stegodera, and Clausilia, form a marked Chinese element. Amphidromus, with 33 species, is the most characteristic land pulmonate. Several genera, whose nucleus of distribution lies among the islands farther east, appear to have penetrated as far as these coasts. Such are Chloritis, Camaena, and Obbina among the Helicidae, Trochomorpha, and, of the operculates, Helicina.
Fig. 206.—Cyclophorus siamensis Sowb., Siam.
Land operculates are very richly developed. In all, there are 17 genera and 104 species[307] known. The tubed operculates attain their maximum, and Cyclophorus is even more abundant than in India. Fresh-water bivalves abound. Dipsas and Pseudodon are common to China, and Unio and Anodonta are profusely represented. A curious resemblance to S. America appears in this group, a single Mycetopus occurring, the only species not Brazilian, while Arconaia appears very closely to approach the Hyria of the same locality. Several genera of the Hydrobia type (Pachydrobia, Jullienia, Chlorostracia) are peculiar.
Land and Fresh-water Mollusca of the Siamese Province
| Streptaxis | 20 |
| Ennea | 3 |
| Helicarion | 7 |
| Microcystis | 3 |
| Sesara (?) | 1 |
| Medyla | 1 |
| Xesta | 4 |
| Macrochlamys | 6 |
| Kaliella | 5 |
| Hyalinia (?) | 1 |
| Hemiplecta | 14 |
| Rhysota | 2 |
| Trochomorpha | 6 |
| Trochomorphoides | 3 |
| Plectopylis | 5 |
| Stegodera | 2 |
| Plectotropis | 12 |
| Trachia | 3 |
| Fruticicola | 2 |
| Acusta | 2 |
| Chloritis | 8 |
| Dorcasia | 1 |
| Camaena | 5 |
| Hadra | 5 |
| Obbina | 1 |
| Amphidromus | 33 |
| Bocourtia | 2 |
| Buliminus | 4 |
| Hypselostoma | 2 |
| Tonkinia | 1 |
| Clausilia | 15 |
| Opeas | 7 |
| Spiraxis (?) | 2 |
| Subulina | 1 |
| Succinea | 4 |
| Vaginula | 7 |
| Limnaea | 7 |
| Planorbis | 6 |
| Canidia | 13 |
| Melania | 39 |
| Faunus | 1 |
| Bithynia | 9 |
| Wattebledia | 1 |
| Stenothyra | 4 |
| Hydrobia | 1 |
| Pachydrobia | 9 |
| Jullienia | 6 |
| Lacunopsis | 6 |
| Chlorostracia | 4 |
| Vivipara | 39 |
| Valvata | 1 |
| Ampullaria | 15 |
| Assiminea | 7 |
| Procyclotus | 6 |
| Dasytherium | 2 |
| Opisthoporus | 5 |
| Rhiostoma | 7 |
| Myxostoma | 1 |
| Pterocyclus | 7 |
| Cyclophorus | 28 |
| Leptopoma | 10 |
| Lagochilus | 6 |
| Pupina | 8 |
| Hybocystis | 3 |
| Alycaeus | 6 |
| Cataulus (?) | 1 |
| Diplommatina | 2 |
| Helicina | 4 |
| Georissa | 2 |
| Modiola (f. w.) | 2 |
| Dreissensia | 3 |
| Anodonta | 17 |
| Mycetopus | 1 |
| Pseudodon | 18 |
| Dipsas | 4 |
| Unio | 64 |
| Arconaia | 1 |
| Cyrena | 6 |
| Batissa | 1 |
| Corbicula | 35 |
(c) The Malay Province includes the peninsula of Malacca south of Tenasserim, and the series of islands beginning with Sumatra and stretching eastward up to the Ké Is., besides Borneo and Celebes. The Philippines form a separate province.
The Malay province is singularly poor in representative forms, whether we regard it as a whole or consider the islands separately. Not a single genus, with the exception of Rhodina (Malacca), appears to be peculiar. The contrast with the West Indies is in this respect very striking. Java, for instance, which is well explored, and almost exactly eleven times the size of Jamaica, has about 100 species of land Mollusca, while Jamaica has about 460.
[308]
This want of individuality in the land Mollusca of the Malay islands is accounted for by a consideration of the sea depths which separate them from the Asiatic mainland. The accompanying map, the red line on which is intended to show what would be the result of an elevation of the sea bottom for no greater amount than 100 fathoms, exhibits clearly the fact that these islands are practically a part of Asia, a large stretch of very shallow sea extending between Siam and the greater part of the north-west coast of Borneo.
In all probability the three great islands of Sumatra, Java, and Borneo were united with the mainland of Asia, and with one another, at a period, geologically speaking, comparatively recent. This follows from the general uniformity of their land Mollusca, both as regards one another and as regards the mainland. Nor do the smaller members of the island series—Bali, Lombok, Sumbawa, Flores, Timor, and Timor Laut—show any marked individuality in the possession of peculiar genera. Wallace’s line is absolutely non-existent, so far as the land Mollusca are concerned. The really noticeable break in distribution comes with the Aru Is., for while the Tenimber group (Timor Laut, etc.) are decidedly Malay, and the Ké Is., in the poverty of our information, uncertain, the Aru Is. are as Papuan as New Guinea itself. The profound depths of the Banda Sea to the north, and the Timor Sea to the south, appear to have kept the islands from Flores to Timor Laut free from the intrusion of any Moluccan or any considerable Australian element. The Moluccas, as has been already remarked, besides possessing considerable peculiarities of their own, unite a mixture of the Malay and Papuan elements, and serve as a sort of debatable ground for the meeting of the two.

Fig. 207.—Ariophanta Rumphii v. d. B., Java.
The Malay peninsula is practically another island of somewhat the same shape and general trend as Sumatra, and about one-half the size. Its general relations—and the remark applies to the great Sunda Islands as well—appear to be rather more with Burmah, Tenasserim, and even the Cingalese district, than with Siam. Points of connexion between Ceylon and Sumatra, and Ceylon and Borneo, have already (p. 304) been brought out.
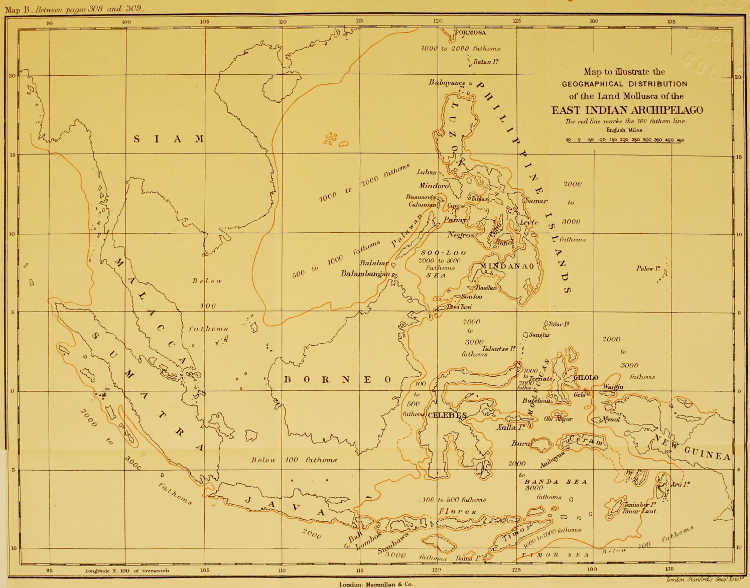
Map to illustrate the
GEOGRAPHICAL DISTRIBUTION
of the Land Mollusca of the
EAST INDIAN ARCHIPELAGO
The red line marks the 100 fathom line
London: Macmillan & Co.
London Stanford’s Geogl. Estabt.
[309]
It seems not impossible, from the point of view of the land Mollusca only, that the Sunda Islands may at one time have stretched much farther into the Bay of Bengal, prolonged, perhaps, into what are now the Andaman and Nicobar groups, while Ceylon and the western side of the Deccan, united into one continuous piece of land, and possibly separated from N. India by a wide stretch of sea, extended farther eastward in a long island, or series of islands.
Java, from its Mollusca, does not appear to hold the comparatively isolated position which its mammals and birds seem to indicate. Borneo, on the other hand, is more Siamese than Java or Sumatra in respect of a group whose metropolis is Siam, namely, the tubed operculates; for while that section is represented by 3 species in Sumatra and only 2 in Java, in Borneo it has as many as 19, Rhiostoma not occurring in the two former islands at all. Alycaeus, Lagochilus, Pupina, and Cyclophorus are found throughout, but Hybocystis (Malacca, 1 sp.) does not quit the mainland. Borneo is remarkably rich in land operculates, especially noticeable being the occurrence (11 sp.) of Opisthostoma (Fig. 208), a most extraordinary form of land shell (Ceylon, Siam), of Diplommatina (17 sp.), and Raphaulus. The occurrence of a single Papuina (Moluccas eastward) is very remarkable.
Fig. 208.—A, Opisthostoma Cookei E. A. Smith, Borneo; B, Opisthostoma grandispinosum G.-A., Borneo. Both × 8.
Amphidromus is a genus characteristic of the great Sunda Islands, attaining its maximum in Java (12 sp.). The Indian Glessula still has one species each in Sumatra, Java, and Borneo. One species of Streptaxis[369] occurs in Malacca, but Ennea (3 sp.) reaches as far east as Borneo and the Philippines. Parmarion, Helicarion, Ariophanta, and other groups of the Naninidae are well represented.[310] Hemiplecta and Xesta are abundant and large, while the Rhysota of Borneo contain some huge sinistral forms. Rhodina is a remarkable form from Malacca, whose exact generic position is not yet settled. Clausilia has a few species on all the islands, the last occurring on Ternate, and a single Papuina (Moluccas and N. Guinea) occurs in Borneo.
The Island of Celebes marks the beginning of a distinct decrease in the Indo-Malay element. The Naninidae lose ground, in proportion to the Helicidae, Macrochlamys, for instance, being represented by only one species, and Hemiplecta by four. Other characteristic genera of the Indian region dwindle, such as Amphidromus, Clausilia, the tubed operculates, and Cyclophorus, while Sitala, Kaliella, Glessula, and Plectotropis disappear altogether. Comparing the total numbers of Naninidae and Helicidae from Sumatra to New Guinea, we obtain this interesting result:—
| Sumatra | Java | Borneo | Celebes | Moluccas | N. Guinea | |
| Nanina (all genera) | 26 | 32 | 51 | 22 | 36 | 40 |
| Helix (all genera) | 7 | 11 | 13 | 14 | 55 | 91 |
It will be noticed that the proportion of Naninidae to Helicidae, which has been nearly 4 to 1 in Sumatra, falls to 3 to 1 in Java, and rises again to 4 to 1 in Borneo (showing the essentially continental character of the island); in Celebes it further falls to 3 to 2, while in the Moluccas the scale turns and Helix has the advantage by about 8 to 5, and in N. Guinea by more than 2 to 1.
Fig. 209.—Amphidromus perversus L., Java.
There is the same absence of marked features of individuality in Celebes as in the islands dealt with above. Not a single genus is peculiar. The nature of the sea bottom between Borneo and Celebes, with its indications of a somewhat broad bridge over an otherwise deep channel of separation, would seem to account for and suggest the true explanation of the facts as they stand. At the same time, there are indications of a certain amount of contrast between N. and S. Celebes. The Indian element, which constitutes the preponderating majority of the fauna, is common to north and south alike. But the north part of the island, in[311] which Obba and Obbina occur, shows decided relationship with the Philippines, while the occurrence of three Chloritis and one Planispira tend to approximate S. Celebes rather with the Moluccas.
The islands eastward of Java, from Bali to Timor Laut and the Tenimber Is., present no trace of individual peculiarities; they simply carry on the Indo-Malay fauna as though along a great peninsula. Even Timor, surrounded as it is on all sides by sea of profound depth, shows no sign of possessing even one peculiar genus. Amphidromus, perhaps the most characteristic of all Indo-Malay genera, occurs throughout, diminishing in numbers as we go eastward (Bali, Lombok, and Sumbawa 4 sp., Timor 2 sp., Timor Laut 1 sp.), while Plectotropis reaches no farther than Flores and Timor. The tubed operculates are altogether wanting. In Timor Laut we have Moluccan influence appearing in 3 Chloritis, and there is one (supposed) Corasia. Two Helices of a marked Australian type (Rhagada) occur, one in Flores, the other on Dama I., south-west of Timor. The configuration of the sea bottom (see map) would lead us to believe that the north-west coast of Australia once stretched a good deal nearer to these islands.
The Moluccas, taken as a whole, constitute a transition region between the Indo-Malay and the Papuan faunas, uniting, to a very considerable extent, the features of both. They fall into two well-defined groups. The northern, or Ternate group, consists of Gilolo (Halmahera), Batchian, and the outlying islands as far south as and including Obi major. The southern, or Amboyna group, consists of Buru, Ceram, Amboyna, and the chain of islands to the south-east of Ceram, as far as, and including the Ké Is.
The Ternate group shows decidedly closer relations with New Guinea than the Amboyna group. Thus, among the Helices, the markedly Papuan genus Papuina is represented by 7 species in the Ternate group, but by 1 in the Amboyna group. Again, the Cristigibba section of Planispira, which is a Papuan form, has 4 representatives in the northern group, but only 1 in the southern. Certain points of connexion with Celebes come out in the southern group which are wanting in the northern; thus of Chloritis there are 8 species in Amboyna, 0 in Ternate, 3 in Celebes.
In the Moluccas the Helicidae, for the first time as we move[312] eastward from India, gain the ascendancy over the Naninidae, the numbers being, Helix 55, Nanina 36. If we take the groups separately, we find that in the Amboyna group the proportion is 22 to 23, while in the Ternate group it is 33 to 13, an additional proof that the Amboyna group is far less Papuan than the Ternate. Of Planispira, the most characteristic sub-genus of Helix, there are 12 species in the Ternate group, and 5 in the Amboyna. The section Phania, which contains 4 species of the finest Helices known, is quite peculiar to the Ternate group. One species of Obbina, a sub-genus markedly Philippine, occurs in each group. Several of the Indo-Malay land operculates (e.g. Ditropis) reach their limit here, and here too we have the last Clausilia (strangely absent from the Amboyna group). Amphidromus is not reported on sufficient authority to warrant its insertion in the list.
Land Mollusca of the Moluccas. (T = Ternate, A = Amboyna[370] group)
| Helicarion | 1 A |
| Euplecta | 1 A |
| Xesta | 6 A, 4 T |
| Macrochlamys | 1 A |
| Lamprocystis | 4 A, 2 T |
| Macrocycloides | 4 A |
| Sitala | 1 A |
| Kaliella | 3 A, 1 T |
| Trochomorpha | 3 A, 3 T |
| Endodonta | 1 A |
| Patula | 1 A |
| Plectotropis | 1 T |
| Eulota | 1 A |
| Chloritis | 8 A |
| Planispira | 5 A, 12 T |
| Cristigibba | 1 A, 4 T |
| Obbina | 1 A, 1 T |
| Phania | 4 T |
| Albersia | 3 T |
| Camaena | 1 T |
| Papuina | 1 A, 7 T |
| Pupa | 3 A |
| Vertigo | 2 A |
| Clausilia | 1 T |
| Opeas | 4 A, 4 T |
| Subulina | 1 A |
| Tornatellina | 1 A |
| Vaginula | 1 A |
| Melania | 18 A, 4 T |
| Faunus | 1 A |
| Vivipara | 1 A |
| Acmella | 1 A |
| Diplommatina | 4 A, 2 T |
| Registoma | 1 T |
| Pupinella | 1 A |
| Callia | 2 A |
| Leptopoma | 4 A, 5 T |
| Lagochilus | 1 A, 1 T |
| Ditropis | 3 A |
| Cyclotus | 4 A, 6 T |
| Omphalotropis | 3 A |
| Georissa | 1 T |
| Helicina | 6 A, 3 T |
(d) The Philippine Province.—In the extraordinarily rich development of their Mollusca, the Philippines form a remarkable contrast with the poverty of the adjacent Malay islands. No less than 727 species of land Mollusca alone are known from the group, amongst which are included some of the finest and handsomest forms yet discovered. The main features of the fauna are Indo-Malay, with the addition of a certain Australasian[313] element, and a remarkable development of individual characteristics.
The principal indigenous feature is the profuse abundance of the genus Cochlostyla, a group of large and elegant land shells, partly helicoid, partly bulimoid in shape, many of the species of which are covered with a curious hydrophanous epidermis. They are in the main of arboreal habits, living in the tops of the enormous forests which cover the greater part of the islands. As many as 247 species, belonging to 15 sub-genera, have been described.

Fig. 210.—Cochlostyla (Chrysalis) mindoroensis Brod., Mindoro, Philippines.
Fig. 211.—Cochlostyla (Orthostylus) Portei Reeve, Luzon. × ⅔.
The distribution of the sub-genera of Cochlostyla on the different islands of the Philippine group affords important evidence on the geological relation of the islands to one another. Thus we find Orthostylus and Hypselostyla occurring in the central islands and S. Luzon, but not in Mindanao or Mindoro; we find Chrysalis peculiar to Mindoro, Prochilus to Mindoro and the Cuyos, Ptychostyla to Luban, all these being sub-genera of very marked characteristics. Six out of the fifteen sub-genera are entirely absent from Mindanao, although occurring on the islands in the immediate vicinity. The little group Tablas-Romblon-Sibuyan are entirely deficient in certain sub-genera which occur on the islands surrounding them on all sides.[371]
[314]
Other forms peculiar to the Philippines are Diaphora, a section of Ennea with a curiously produced mouth, and several sub-genera of the Naninidae (Vitriniconus, Vitrinoidea, Hemitrichia). The great Rhysota here find their metropolis. Another very marked group of Helix is Obbina, 19 of the 25 known species being peculiar.
The Helicidae proper of the Philippines are still held in check, as in the greater part of the Indian region, by the Naninidae. The single Trachia and Plectotropis, and the 2 species each of Plectopylis and Satsuma, indicate affinities with Indo-China. Further important Indian relationships are seen in the great Nanina and Cyclophorus, which here attain almost Indian dimensions; in Kaliella (8 sp.), Sitala (2), Clausilia (1). Among the operculates we still have 1 Alycaeus and 1 Coptochilus. Singularly enough, several Indian genera which occur here are not found in the intervening islands of Borneo, Sumatra, or Java, e.g. Streptaxis, Hypselostoma, Ditropis, Acmella, and Cyathopoma. The curiously tubed Malay operculates, Opisthoporus, etc., fail to reach the Philippines proper, although occurring in Borneo and N. Celebes; one of them reaches Palawan. The strikingly Malay genus Amphidromus reaches Palawan, but no farther (1 sp.), while 2 species reach Mindanao, and one of these penetrates as far as Bohol and S. Leyte. Amongst the slugs, Mariaella occurs again only in the Seychelles, and Tennentia only in Ceylon.
Fig. 212.—Helix (Obbina) rota Brod., Philippines.
Land and Fresh-water Mollusca of the Philippines
| Streptaxis | 1 |
| Ennea | 10 |
| Mariaella | 3 |
| Tennentia | 1 |
| Helicarion | 21 |
| Vitrinopsis | 5 |
| Vitrinoidea | 1 |
| Rhysota | 17 |
| Trochonanina | 2 |
| Euplecta | 28 |
| Hemiplecta | 11 |
| Hemitrichia | 15 |
| Xesta | 2 |
| Macrochlamys | 5 |
| Microcystis | 3 |
| Lamprocystis | 17 |
| Bensonia | 4 |
| Vitriniconus | 16 |
| Sitala | 2 |
| Kaliella | 8 |
| Trochomorpha | 21 |
| Endodonta | 1 |
| Plectopylis | 3 |
| Plectotropis | 1 |
| Aulacospira | 3 |
| Pupisoma | 1 |
| Satsuma | 2 |
| Dorcasia | 2 |
| Chloritis | 7 |
| Obbina | 19 |
| Papuina | 1 |
| Phoenicobius | 7 |
| Cochlostyla | 247 |
| Amphidromus | 2 |
| Hapalus (?) | 4 |
| Hypselostoma | 1 |
| Pupa | 4 |
| Clausilia | 1 |
| Subulina | 3 |
| Prosopeas | 2[315] |
| Opeas | 4 |
| Geostilbia | 1 |
| Tornalellina | 1 |
| Succinea | 3 |
| Vaginula | 2 |
| Ancylus | 1 |
| Limnaea | 3 |
| Planorbis | 3 |
| Physa | 2 |
| Melania | 50 |
| Pirena | 2 |
| Bithynia | 1 |
| Vivipara | 7 |
| Ampullaria | 5 |
| Acmella | 2 |
| Diplommatina | 41 |
| Arinia | 6 |
| Pupina | 5 |
| Registoma | 7 |
| Hargreavesia | 1 |
| Callia | 2 |
| Pupinella | 3 |
| Helicomorpha | 4 |
| Coptochilus | 1 |
| Alycaeus | 1 |
| Leptopoma | 42 |
| Lagochilus | 11 |
| Cyclophorus | 31 |
| Ditropis | 7 |
| Cyathopoma | 5 |
| Cyclotus | 19 |
| Omphalotropis | 3 |
| Helicina | 18 |
| Georissa | 3 |
| Anodonta | 1 |
| Cyrena | 3 |
| Corbicula | 7 |
Islands adjacent to the Philippines.—The Philippines are connected with Borneo by two distinct ridges or banks of elevation, which enclose between them the Soo-loo or Mindoro Sea. There can be little doubt that these ridges represent the ancient highway of transit, by which Indo-Malay species passed into the Philippines. The depth of the sea on either side is profound, ranging from an average of about 1000 fathoms west of Palawan to 2550 off the south-west coast of Mindanao.
It appears that the fauna of the Soo-loo ridge is definitely Philippine up to and including Bongao, Sibutu, and Bilatan, the last islands at the Bornean end of the ridge. On these are found two species of Cochlostyla and an Obbina.
The Palawan ridge may also be described as more or less Philippine throughout. One species of Cochlostyla occurs on Balabac, just north of Borneo, and two on Palawan, but these are perhaps counterbalanced by the definitely Indo-Malay Amphidromus and Opisthoporus (1 sp. each). At the northern end of the ridge, on Busuanga and Calamian, the Philippine element predominates.
Representatives of two remarkable groups of Helix (Camaena and Phoenicobius) occur along the Palawan ridge and in Mindoro. The Phoenicobius find their nearest allies in the curious small group known as Obba, from N. Celebes, the Camaena possibly in a type of Helix (Hadra) occurring in New Guinea and N.E. Australia. The only other Helix from the whole of the E. Indies which bears any resemblance to the Phoenicobius group is H. codonodes Pfr., which is peculiar to the Nicobars. A few forms assigned to Camaena also occur in Further India and Siam. It would appear possible, therefore, that these two isolated groups are a sort of survival of a fauna which perhaps had once a much more extended range.
[316]
(2) The Chinese Sub-region.—The Chinese Sub-region includes the whole of China from its southern frontier up to and including the basin of the Blue or Yang-tse River, together with the coast district, including Corea, perhaps as far north as Vladivostok, and the outlying islands of Hainan, Formosa, the Loo-Choo and Bonin groups, and Japan to the north of Niphon. It may be divided into two provinces, the Chinese and the Japanese.
(a) The fauna of the Chinese province proper bears, in many respects, strong marks of relationship to that of India and Siam. Thus Streptaxis, Helicarion, Macrochlamys, Kaliella, Sitala, Ariophanta, Rhysota, Hemiplecta, Diplommatina, Opisthoporus, Pterocyclus, Lagochilus, and Alycaeus all occur, especially in Southern China. The two points in which the sub-region bears special marks of individuality are Helix and Clausilia. The sub-genera of Helix which have their metropolis in China are Satsuma, Cathaica, Aegista, Acusta, Euhadra, Plectotropis, and Plectopylis. Sinistral forms (compare Fig. 213) are rather prevalent. In several cases—e.g. Trichia, Gonostoma, Fruticicola—there is a reappearance of forms which appear to belong to well-known European sub-genera. Clausilia here attains a kind of second centre of distribution, and is represented by its finest forms, which belong to several peculiar sub-genera. The carnivorous Mollusca are not abundant, and are represented by Rathouisia (a peculiar genus of naked slug), Ennea, and Streptaxis. In the western provinces Buliminus is abundant in several sub-genera, one of which appears to be the European Napaeus.
Fig. 213.—Helix (Camaena) cicatricosa Müll., China.
There is little which is striking in the operculates, which are most abundant in the south, and appear to be mainly derived from Indian and Siamese sources. The occurrence of Helicina (3 sp.), Omphalotropis (1), Leptopoma (2), and Realia (2), is evidence of some influence from the far East. Heudeia is a very remarkable and quite peculiar form of Helicina with internal plicae, perhaps akin to the Central American Ceres.
Fresh-water genera are exceedingly abundant, especially Melania, Unio, and Anodonta. The occurrence of Mycetopus (a South-American genus) is remarkable. There are several[317] peculiar forms of fresh-water operculates, whose exact position is hardly yet assured.
Land and Fresh-water Mollusca of the Chinese Province
| Rathouisia | 1 |
| Streptaxis | 7 |
| Ennea | 12 |
| Parmarion | 2 |
| Helicarion | 15 |
| Euplecta | 3 |
| Macrochlamys | 19 |
| Microcystina | 2 |
| Microcystis | 7 |
| Kaliella | 16 |
| Sitala | 8 |
| Ariophanta | 1 |
| Rhysota | 5 |
| Hemiplecta | 1 |
| Trochomorpha | 2 |
| Limax | 1 |
| Philomycus | 1 |
| Patula | 2 |
| Gonostoma | 4 |
| Metodontia | 2 |
| Vallonia | 1 |
| Plectotropis | 9 |
| Fruticicola | 11 |
| Satsuma | 14 |
| Trichia | 10 |
| Cathaica | 22 |
| Aegista | 10 |
| Armandia | 3 |
| Acusta | 15 |
| Obbina | 1 |
| Camaena | 5 |
| Euhadra | 14 |
| Plectopylis | 19 |
| Stegodera | 6 |
| Chloritis | 1 |
| Hel. Inc. sed. | 39 |
| Buliminus | 21 |
| Buliminopsis | 3 |
| Buliminidius | 3 |
| Napaeus | 14 |
| Rachis (?) | 4 |
| Pupa | 10 |
| Clausilia | 102 |
| Opeas | 12 |
| Euspiraxis | 1 |
| Subulina | 5 |
| Stenogyra (?) | 12 |
| Succinea | 8 |
| Vaginula | 7 |
| Limnaea | 2 |
| Planorbis | 6 |
| Melania | 44 |
| Paludomus | 3 |
| Bithynia | 12 |
| Lithoglyphus | 3 |
| Melantho (?) | 1 |
| Pachydrobia | 1 |
| Prososthenia | 2 |
| Stenothyra | 2 |
| Hydrobia | 2 |
| Mecongia | 1 |
| Oncomelania | 9 |
| Margaracya | 1 |
| Rivularia | 4 |
| Delavaya | 1 |
| Fenouillia | 1 |
| Vivipara | 34 |
| Diplommatina | 20 |
| Pupina | 6 |
| Alycaeus | 23 |
| Leptopoma | 2 |
| Lagochilus | 10 |
| Cyclophorus | 18 |
| Coelopoma | 1 |
| Pterocyclus | 3 |
| Opisthoporus | 4 |
| Cyclotus | 10 |
| Scabrina | 4 |
| Ptychopoma | 12 |
| Omphalotropis | 1 |
| Realia | 2 |
| Pseudopomatias | 1 |
| Helicina | 3 |
| Georissa | 4 |
| Heudeia | 1 |
| Cyclas | 1 |
| Corbicula | 50 |
| Unio | 53 |
| Monocondylaea | 1 |
| Anodonta | 55 |
| Mycetopus | 12 |
| Pseudodon | 1 |
| Dipsas | 4 |
The island of Hainan, in the extreme south of the sub-region, has 40 species of Mollusca, 22 of which are peculiar, but there is no peculiar genus.
The Mollusca of Formosa, although in many cases specifically distinct, show close generic relationship with those of China. The characteristic Chinese groups of Helix and Clausilia occur, and there is still a considerable Indian element in several species of Streptaxis, Macrochlamys, Kaliella, and Alycaeus. The occurrence of two Amphidromus, a genus which, though Siamese, is not found in China or Hainan, is remarkable.
The peninsula of Corea must undoubtedly be included in the Chinese sub-region. It is true that the land operculates scarcely occur, but there are still a number of Clausilia, and several of the characteristic Chinese groups of Helix are reproduced. In some points Corea appears to show more affinity to Japan than[318] to China, four of the Helices being specifically identical with those of Japan, but the peninsula is at present too little explored for any generalisations to be made as to its fauna in this respect.
(b) Japanese Province.—Kobelt distinguishes four groups of Mollusca inhabiting Japan (a) circumpolar species, actually occurring in Europe, Siberia, or N. America, or represented by nearly allied species (these of course do not belong to the Japanese province as such); (b) Indo-tropical species; (c) species which are Chinese or akin to Chinese; (d) peculiar species, a mixture of two forms, southern and northern, the latter being chiefly Hyalinia, Patula, and Fruticicola. Out of a total of 193 Japanese species, at least 164 are peculiar.
The Japanese Helices belong to sub-genera common to China (Plectotropis 8, Euhadra 21, Acusta 23?); but the Naninidae scarcely occur at all. The principal feature of the fauna is the development of Clausilia, which presents some extraordinarily fine forms. One slug (Philomycus) is identical with an Indian species. The operculates, which consist mainly of a few species each of Diplommatina, Cyclophorus, Pupinella, Pupina, Helicina, and Georissa, belong almost exclusively to the southern islands Kiu-siu, Sikoku, and southern Niphon. The three species usually reckoned as Japonia are probably forms of Lagochilus.
This region includes all the islands of the Pacific east of the Moluccas, and falls into three sub-regions—the Papuan, the Australian, and the Polynesian.
1. The Papuan Sub-region may be divided into—(a) the Papuan Province proper, which includes New Guinea, with the Aru Is. and Waigiou, the Admiralty Is., New Ireland, New Britain, and the d’Entrecasteaux and Louisiade Groups; (b) the Queensland Province, or the strip of N.E. Australia from C. York to the Clarence R. (about 29° S. lat.); (c) the Melanesian Province, which includes the New Hebrides, New Caledonia, with the Loyalty Is. and the Viti Is. The Solomons form a transition district between the Papuan and Melanesian provinces, abounding on the one hand in characteristic Papuan Helices, while on the other they form the north-western limit of[319] Placostylus, the group especially characteristic of the Melanesian province.
(a) The Papuan Province.—The molluscan fauna of New Guinea is the richest and by far the most original of all the Australasian region. We find ourselves, almost in a moment, in a district full of new and peculiar forms. New Guinea may be regarded as the metropolis of the rich Helicidan fauna, which is also characteristic of the Moluccas to the west, of N. and N.E. Australia to the south and south-east, and of the Solomons and other groups to the north-east. Here abound species of Papuina and Insularia (the latter being quite peculiar), among which are found, if not the largest, certainly the most finished forms of all existing Helices. Chloritis (13 sp.), Planispira (5), and Cristigibba (9) are common with the Moluccas, while a tropical Australian element is shown in Pedinogyra (1) and Hadra (4). Very remarkable, too, is the occurrence of one species of Obbina and Rhysota, genera which culminate in the Philippines and here find their most eastward extension; while a single Corasia serves to form a link between the Corasia of the Philippines and those of the Solomon Is., if the latter are true Corasia.
We naturally find considerable traces of a Polynesian element, which appears to be principally characteristic of the eastern part of the island. Most noteworthy in this respect is the occurrence of Partula (3), Tornatellina (1), Charopa (1), Thalassia (3). As compared with the true Pulmonata, the operculates are feebly represented, and the great majority are of a markedly Polynesian type. Not a single Cyclophorus occurs; Lagochilus, Alycaeus, and all the tubed operculates, so marked a feature of the Indo-Malay fauna, are conspicuous by their absence, and the prevailing genera are Cyclotus, Helicina, and a number of sections of Pupina. Leptopoma, as in the Philippines, is strongly represented. Not that an Indo-Malay element is altogether absent. We still have Xesta (5), Hemiplecta (8), and even Sitala (2), but the great predominance of Helix seems to have barred the progress, for the greater part, of the Indian Naninidae.
The slugs appear to be represented by a solitary Vaginula. A single Perrieria is a very marked feature of union with Queensland, where the only other existing species (P. australis) occurs. The solitary Rhytida, so far the only representative of the carnivorous group of snails, emphasises this union still[320] further. Little is known of the fresh-water fauna. Melania (28 sp.) is predominant, but on the whole the relations are Australian rather than Indo-Malay. Ampullaria is wanting, while a decisive point of similarity is the occurrence of Isidora (3 sp.), a genus entirely strange to the Oriental region, but markedly characteristic of the Australasian.
Land and Fresh-water Mollusca of New Guinea
| Rhytida | 1 |
| Helicarion | 2 |
| Rhysota | 1 |
| Hemiplecta | 11 |
| Xesta | 2 |
| Microcystis | 3 |
| Microcystina | 2 |
| Sitala | 2 |
| Oxytes (?) | 2 |
| Conulus | 1 |
| Trochomorpha | 8 |
| Nanina (?) | 3 |
| Charopa | 1 |
| Thalassia | 3 |
| Ochthephila(?) | 1 |
| Chloritis | 13 |
| Planispira | 5 |
| Cristigibba | 9 |
| Insularia | 17 |
| Obbina | 1 |
| Albersia | 3 |
| Hadra | 4 |
| Pedinogyra | 1 |
| Papuina | 35 |
| Corasia (?) | 1 |
| Bulimus (?) | 1 |
| Calycia | 4 |
| Partula | 3 |
| Pupa | 1 |
| Stenogyra | 1 |
| Tornatellina | 1 |
| Perrieria | 1 |
| Succinea | 1 |
| Vaginula | 1 |
| Limnaea | 2 |
| Isidora | 3 |
| Melania | 28 |
| Faunus | 1 |
| Vivipara | 4 |
| Diplommatina | 1 |
| Pupina | 4 |
| Pupinella | 3 |
| Omphalotropis | 2 |
| Bellardiella | 2 |
| Leptopoma | 16 |
| Cyclotus | 5 |
| Cyclotropis | 5 |
| Helicina | 15 |
| Unio | 4 |
| Cyrena | 3 |
| Corbicula | 1 |
| Batissa | 8 |
Waigiou is practically a part of New Guinea. Twelve genera and twenty species of Mollusca are known, eight of the latter being peculiar. The occurrence of Papuina, Insularia, and Calycia sufficiently attest its Papuan relationship. Two species each of Albersia, Chloritis, and Planispira occur.[372]
The Aru Is. are, as we should expect from their position, and particularly from the configuration of the adjacent sea bottom (see map), markedly Papuan. At the same time they show unmistakable signs of long-continued separation from the parent island, for of their 36 land Mollusca 15, and of their 20 fresh-water Mollusca 9 are peculiar. The Papuan element consists in the presence of Papuina, Albersia, and Cristigibba. Moluccan influence is not absent, for the three Helicina, the Albersia, and one Cyclotus are all Moluccan species. The fresh-water fauna appears to be a mixture of varied elements. The single Segmentina is common to India, the Glaucomya to Malacca and the Philippines, while the single Batissa is also found in New Zealand.
[321]
Land and Fresh-water Mollusca of the Aru Islands
| Xesta | 4 |
| Microcystis | 1 |
| Hyalinia(?) | 1 |
| Trochomorpha | 1 |
| Patula | 1 |
| Eulota | 1 |
| Chloritis | 5 |
| Cristigibba | 2 |
| Albersia | 1 |
| Papuina | 4 |
| Pupa | 2 |
| Stenogyra | 2 |
| Planorbis | 1 |
| Segmentina | 1 |
| Melania | 14 |
| Leptopoma | 3 |
| Moussonia | 1 |
| Realia | 1 |
| Cyclotus | 3 |
| Helicina | 3 |
| Cyrena | 2 |
| Glaucomya | 1 |
| Batissa | 1 |
The Louisiades, the d’Entrecasteaux, and Trobriand Is., and Woodlark I., are closely related to New Guinea, containing no peculiar genera. Each group, however, contains a considerable proportion of peculiar species, an indication that their separation from New Guinea dates from a very distant period. From the Louisiades are known 34 species in all, 22 of which are peculiar.
The fauna of the Admiralty Is., of New Hanover, and New Ireland is markedly Papuan, without any especial feature of distinction. The Admiralty Is. contain 15 sp. Papuina, 7 Chloritis, 1 Planispira, and 1 Corasia. A single Janella shows relationship with the New Hebrides and with New Zealand. In New Ireland Planispira (which is specially characteristic of W. New Guinea and the Moluccas) has disappeared, but there are 7 Papuina and 6 Chloritis. The essentially Polynesian Partula is present in both groups.
The prominent feature of the Mollusca of the Solomon Is. is the extraordinary development of Papuina, which here culminates in a profusion of species and singularity of form. The genus is arboreal, crawling on the branches and attaching itself to the leaves of trees and underwood. Of the 140 land Pulmonata known from the group, no less than 50, or 36 per cent, are Papuina. Ten species of Corasia occur, but whether the shells so identified are generically identical with those of the Philippines, is not satisfactorily determined. Trochomorpha, with 22 species, here attains its maximum. Chloritis begins to fail, but still has 3 species. Indo-Malay influence still appears, though feebly, in Hemiplecta (3), Xesta (1), and possibly even Macrochlamys (1). The Rhytida, the 3 Hadra, and possibly the Paryphanta represent the Australian element. The growing numbers of Partula (13), the small and inconspicuous land operculates (only 22 in all, with Helicina very prominent), and the almost complete absence of fresh-water bivalves, show signs of strong Polynesian affinities. An especial link with the New[322] Hebrides, New Caledonia, and the Viti Is. is the occurrence of Placostylus (16 sp.). It is very remarkable that this genus should occur in the Solomon Is. and not in New Ireland. The occurrence of Streptaxis, if authentic, is very noteworthy, the nearest species being from the Philippines.
Land and Fresh-water Mollusca of the Solomon Islands
| Streptaxis (?) | 1 |
| Rhytida | 1 |
| Paryphanta (?) | 1 |
| Helicarion | 2 |
| Xesta | 1 |
| Macrochlamys | 1 |
| Hemiplecta | 3 |
| Microcystis | 2 |
| Trochomorpha | 22 |
| Nanina (?) | 2 |
| Patula | 1 |
| Thalassia | 2 |
| Chloritis | 3 |
| Philina | 2 |
| Hadra | 3 |
| Papuina | 50 |
| Merope | 1 |
| Corasia (?) | 10 |
| Placostylus | 16 |
| Partula | 13 |
| Succinea | 1 |
| Melania | 18 |
| Diplommatina | 2 |
| Pupina | 4 |
| Leptopoma | 4 |
| Omphalotropis | 2 |
| Cyclotus | 1 |
| Cyclotropis | 2 |
| Helicina | 7 |
| Unio | 1 |
(b) The Queensland Province.—The strip of coast-line from Cape York to the Clarence R. stands apart from the rest of Australia, and is closely connected with New Guinea. There can be little doubt that it has been colonised from the latter country, since an elevation of even 10 fathoms would create (see map) a wide bridge between the two. Many of the genera are quite strange to the rest of Australia. Land operculates are abundant, and of a Papuan type. Several of the characteristic Papuan genera of Helix (Papuina, Chloritis, Planispira) occur, while Hadra attains its maximum. Panda, Pedinogyra, and Thersites are three remarkable groups in a rich Helix fauna. Parmacochlea is a peculiar form akin to Helicarion. The carnivorous Mollusca are represented by Rhytida, Diplomphalus (New Caledonia), and Elaea. One species of Janella, a slug peculiar to this region, occurs. The predominant fresh-water genus is Bulinus (Isidora). Ampullaria and Anodonta are entirely absent from Australia and New Zealand.
Fig. 214.—Characteristic Australian Helices: A, H. (Hadra) pomum Pfr.; B, H. (Thersites) richmondiana Pfr. × ⅔.
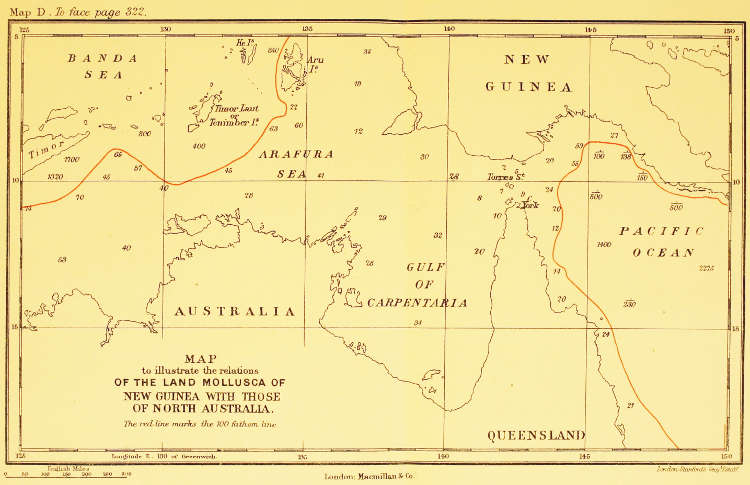
Map D. To face page 322.
MAP
to illustrate the relations
OF THE LAND MOLLUSCA OF
NEW GUINEA WITH THOSE
OF NORTH AUSTRALIA.
The red line marks the 100 fathom line
London: Macmillan & Co.
[323]
Land Mollusca of the Queensland Province
| Diplomphalus | 1 |
| Rhytida | 10 |
| Elaea | 1 |
| Parmacochlea | 1 |
| Helicarion | 7 |
| Nanina | 3 |
| Hyalinia | 10 |
| Thalassia | 4 |
| Charopa | 5 |
| Patula (?) | 4 |
| Macrocyclis (?) | 1 |
| Helicella | 10 |
| Planispira | 8 |
| Hadra | 51 |
| Chloritis | 5 |
| Pedinogyra | 1 |
| Thersites | 1 |
| Papuina | 6 |
| Panda | 2 |
| Helix (inc. sed.) | 6 |
| Bulimus (?) | 1 |
| Stenogyra | 1 |
| Tornatellina | 4 |
| Pupa | 3 |
| Vertigo | 4 |
| Perrieria | 1 |
| Succinea | 3 |
| Vaginula | 1 |
| Janella | 1 |
| Georissa | 1 |
| Pupina | 16 |
| Hedleya | 1 |
| Callia | 1 |
| Diplommatina | 3 |
| Ditropis | 2 |
| Dermatocera | 1 |
| Helicina | 8 |
(c) The Melanesian Province includes those islands on which the remarkable group Placostylus occurs, the metropolis of whose distribution is New Caledonia. These islands are very possibly the remains of what was once a much wider extent of land. A single species of Placostylus occurs both on Lord Howe’s I. and in the North I. of New Zealand, but this fact, while highly interesting as indicating a possible former extension of land in a south-easterly direction, is hardly sufficient to bring these islands within the province as now limited. The Solomon Is., although containing Placostylus as far to the west as Faro I., form, as has been already stated, a transitional district to the Papuan province.
New Caledonia.—The chief features of the Mollusca are the remarkable development of the helicoid carnivorous genera Rhytida (30 sp.) and Diplomphalus (13 sp.), and of Placostylus (45 sp.). There is a stray Papuina, and a peculiar form Pseudopartula, but Helix has almost entirely disappeared. Polynesian influence is represented by Microcystis (3 sp.), the so-called Patula (13 sp., many of which are probably Charopa), Tornatellina (2 sp.), and Helicina (20 sp.). Partula does not reach so far south, but there are two species of Janella. The recurrence of Melanopsis (19 sp.), absent from the whole Oriental region, is curious, and forms another link with New Zealand. The curious sinistral Limnaea (Isidora), common with Australia and New Zealand, is abundant.
Fig. 215.—Placostylus caledonicus Pet., New Caledonia, × ⅔.
The New Hebrides link New Caledonia and the Solomons by[324] their possession of the typical heavy Placostylus (5 sp.) of the former, and the lighter and more elegant Charis (2 sp.) of the latter. There are 4 Papuina, and Partula is abundant (18 sp.), but there is no evidence at present that the carnivorous genera or the Melanopsis and Isidora of New Caledonia occur.
The Fiji Is., by the possession of 14 Placostylus of the Charis section, which is entirely absent from the adjacent Tonga group, form the eastern limit of the province. There appears to be only a single Partula, but the Polynesian element, especially as seen in Navicella (8 sp.), Neritina (20 sp.), Helicina (11 sp.), and Omphalotropis (11 sp.), is very strong. The Microcystis (9 sp.) and Trochomorpha (14 sp.) are also of a Polynesian type.
(2) The Australian Sub-region includes the whole of Australia (with the exception of the Queensland province) and Tasmania, with New Zealand and the off-lying islands. The fauna, from the prevalence of desert, is scanty, especially in genera. Land operculates are almost entirely wanting. Limax is not indigenous, though several species have become naturalised. The bulk of the fresh-water species belong to Isidora, and it is doubtful whether Physa occurs at all. Unio has a few species, and also Vivipara, but neither Anodonta nor Ampullaria occur. There are a few Melania and Neritina.
Tropical South Australia.—The Mollusca are scanty, and occur chiefly in the neighbourhood of the rivers, the soil being arid, with no shelter either of trees or rocks. Fresh-water species predominate, and the rich land fauna of Queensland is totally wanting. There are no land operculates, 6 Hadra, 1 Bulimus (?), 1 Stenogyra.
West Australia.—Owing to the deserts which bound it, the Mollusca are very isolated, only one species being common with N., S., and E. Australia. The chief characteristics are Liparus, a form intermediate between Helix and Bulimus, and, among the Helices, the group Rhagada. There are no slugs, no carnivorous snails, and only three land operculates.
Land Mollusca of West Australia
| Lamprocystis | 1 |
| Hyalinia | 1 |
| Patula | 7 |
| Chloritis | 2 |
| Gonostoma | 2 |
| Trachia | 3 |
| Xerophila | 1 |
| Rhagada | 8 |
| Hadra | 5 |
| Liparus | 10 |
| Pupa | 4 |
| Succinea | 3 |
| Cyclophorus | 2 |
| Helicina | 1 |
[325]
In Eastern and Southern Australia (New South Wales, Victoria, and South Australia) the tropical element, so abundant in Queensland, almost entirely disappears, the last operculate (a Helicina) only reaching Port Macquarie, though several species of Helicarion occur in the extreme south. Hadra is still abundant in New South Wales (18 sp.) and S. Australia (10 sp.), but becomes scarce in Victoria (2 sp.); New South Wales has also one Panda and two Thersites. Cystopelta is common with Tasmania, and one of the Janellidae (Aneitea) with Queensland. The carnivorous snails are represented by Rhytida. Caryodes, a bulimoid group perhaps akin to Liparus, is common with Tasmania only.
Tasmania.—About 80 species of land Mollusca are known, not more than 10 being common with Australia. No land operculates occur; Endodonta and Charopa are rare, and Hadra has entirely disappeared, but Pupa and Succinea occur. Carnivorous genera are represented by Paryphanta, Rhytida, and Rhenea. Anoglypta is a peculiar section of Helix, while Caryodes, Cystopelta, and Helicarion are common with Australia. Among the fresh-water Mollusca are a Gundlachia (see p. 345), and some forms of Amnicola or Hydrobia, one of which (Potamopyrgus) is common only with New Zealand.[373]
The Neozealanian Province.—The Mollusca of New Zealand, with the Kermadec, Chatham, and Auckland Is., are remarkably isolated. Such genera as Nanina, Partula, Pupa, Stenogyra, Succinea, Vaginula, Truncatella, Helicina, and Navicella, which might have been expected to occur, are entirely absent. The bulk of the land Mollusca are small and obscure forms, perhaps remains of a very early type, and appear to belong to the Zonitidae, neither Patula nor Helix occurring at all. The carnivorous forms are represented by Schizoglossa, a peculiar genus akin to Daudebardia, by Paryphanta, an extraordinary group of large shells with a thick leathery epidermis, and by Rhytida and Rhenea. In spite of its extreme isolation, the general relations of the fauna are partly with New Caledonia, partly with E. Australia. The occurrence of Placostylus has already been mentioned (p. 323), and three species of Janella, a genus which also occurs in Queensland and New Caledonia, indicate the same[326] affinity. Otoconcha is peculiar. The fresh-water Mollusca, besides the Isidora characteristic of the sub-region, are partly related to New Caledonia through the occurrence of Melanopsis, partly to Tasmania through Potamopyrgus, while the peculiar Latia is possibly akin to Gundlachia (Tasmania). The land operculates number only 5 genera and 14 species in all, excluding a doubtful Diplommatina.[374]
Land and Fresh-water Mollusca of the Neozealanian Province
| Schizoglossa | 1 |
| Paryphanta | 5 |
| Rhytida | 6 |
| Rhenea | 2 |
| Helicarion | 1 |
| Otoconcha | 1 |
| Microcystis | 1 |
| Trochonanina | 1 |
| Phacussa | 3 |
| Thalassohelix | 5 |
| Gerontia | 2 |
| Allodiscus | 10 |
| Pyrrha | 1 |
| Therasia | 7 |
| Phenacohelix | 3 |
| Suteria | 1 |
| Flammulina | 13 |
| Laoma | 23 |
| Endodonta | 10 |
| Charopa | 28 |
| Placostylus | 1 |
| Carthaea | 1 |
| Tornatellina | 1 |
| Janella | 3 |
| Latia | 2 |
| Ancylus | 2 |
| Limnaea | 5 |
| Amphipeplea | 2 |
| Planorbis | 1 |
| Isidora | 7 |
| Melanopsis | 2 |
| Potamopyrgus | 4 |
| Paxillus | 1 |
| Lagochilus | 7 |
| Omphalotropis | 1 |
| Realia | 4 |
| Hydrocena | 1 |
| Unio | 9 |
| Sphaerium | 1 |
| Pisidium | 2 |
Lord Howe’s I. is remarkable as containing a Placostylus, which thus links the island with this province. The remainder of the fauna is Polynesian, with the exception of a species (common to the Fijis) of Parmella, a slug akin to Helicarion, Parmacochlea, and Cystopelta.
(3) The Polynesian Sub-region includes all the island groups of the central and southern Pacific (except those classified in the Papuan and Australian sub-regions), from the Pelews and Carolines in the west to the Marquesas and Paumotus in the east, and from the Tonga group in the south to the Sandwich Is. in the north. It may be subdivided into (a) the Polynesian province proper, and (b) the Hawaiian province, which includes the Sandwich Is. only.
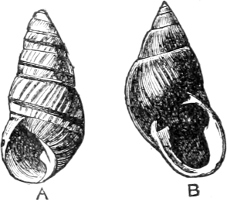
Fig. 216.—Characteristic Polynesian Mollusca: A, Achatinella vulpina Fér., Sandwich Is.; B, Partula planilabrum Pease, Society Is.
(a) The general features of the Polynesian province are very similar throughout, although the Mollusca of each island group are in the main peculiar. The species are mostly small[327] and obscure. Helix scarcely occurs, its place being taken by small Zonitidae (Microcystis, Charopa, Trochomorpha, etc.), and by groups of so-called Patula (Endodonta, Pitys, etc.), the exact position of which is not yet settled. Libera, remarkable for its method of ovipositing (p. 128), is peculiar to the Society and Hervey Is.; Partula is almost universal, attaining its maximum (40 sp.) in the Society Is.; Tornatellina, Pupa, and Vertigo occur throughout.
The land operculates consist chiefly of Omphalotropis, Pupina, Realia, and Helicina. Diplommatina and Palaina are abundant on the Pelews, and a Moussonia occurs in the Samoa Is. Ostodes, a small form of Cyclophorus, is found in some of the southern groups. The fresh-water operculates are Melania, Neritina (including Clithon, a sub-genus furnished with spines), and Navicella; there are no Unionidae, while fresh-water Pulmonata are very scarce.
(b) The land Mollusca of the Hawaiian province are distinguished by the possession of four entirely peculiar genera—Achatinella, Leptachatina, Carelia, and Auriculella. More than 300 of the two former genera have been described, every mountain valley of some of the islands having its own peculiar species. The destruction of the indigenous herbage by goats is rapidly extinguishing many forms. Partula, and the small land operculates, so characteristic of the other groups, are, with the exception of Helicina, entirely wanting. The occurrence of one of the Merope group of Helix (Solomon Is.) is remarkable, and there is a rich development of Succinea. “Patula,” Microcystis, Tornatellina, and the other small Polynesian land Pulmonata are well represented. The presence of Isidora, absent from the central Pacific groups, is remarkable, and Erinna is a peculiar genus belonging to the Limnaeidae.
[328]
The Ethiopian region includes the whole of Africa south of the Great Desert, and Southern Arabia, together with the outlying islands, excepting those of the Atlantidean province (p. 297).
Regarded as a whole, the Ethiopian is poorest in land Mollusca of all the tropical regions. And yet its characteristics are very remarkable. The entire Achatina group is peculiar, and takes, especially in W. Africa, some curious forms (Columna, Perideris, Pseudachatina). Carnivorous Mollusca (Ennea, Gibbus, etc.) are highly developed, especially in the south and east, the largest known helicoid form (Aerope) being from Natal. In the possession of these types of the Agnatha, Africa is more closely related to the Australasian than to the Oriental region. The true Cyclostoma are entirely peculiar to the region, but are absent from West Africa.
Fresh-water Mollusca are abundant and characteristic, especially in and near the Great Lakes. Lanistes, Cleopatra, and Meladomus, among the operculates, together with Mutela and Aetheria (Unionidae), Galatea and Fischeria (Cyrenidae), are peculiar.
In its negative, as well as its positive features, the Ethiopian region is markedly isolated. Helicidae and Naninidae are equally deficient, the former, indeed, attaining some numerical predominance in the extreme south, but the species are nearly all insignificant in size and colouring. It is only in Madagascar that Helix asserts itself. Arion, Limax, Hyalinia, Clausilia, and a[329] number of other genera abundant along the Mediterranean, are either altogether absent, or are very scantily represented. Land operculates, so characteristic of other tropical countries, are almost entirely wanting. If we disregard the Malagasy sub-region, there are scarcely forty species of land operculates on the whole African continent.
The Ethiopian region may be divided into three sub-regions: (1) the Central African; (2) the South African; (3) the Malagasy.
(1) The Central African Sub-region is bounded on the north by the Great Desert, on the east and west by the ocean, and on the south by a line roughly drawn between the mouth of the Orange River and Delagoa Bay; it also includes S. Arabia. No natural features exist which tend to break up this vast district into areas of independent zoological development. The absence of long and lofty mountain ranges, the enormous size of the great river basins, and the general uniformity of climate, equalise the conditions of life throughout. It will be convenient to break the sub-region up into provinces, but in most cases no precise line of demarcation can be laid down.
(a) The Senegambian Province may be regarded as extending from the mouth of the Senegal River to Cape Palmas. Only 8 genera of land Mollusca are known, including 4 Limicolaria and 3 Thapsia, with 1 small Cyclophorus. Fresh-water genera are abundant, and include most of the characteristic Ethiopian forms.
(b) The West African Province extends from Cape Palmas to the mouth of the Congo, and is rich in Mollusca. The great Achatina, largest of land snails, whose shell sometimes attains a length of 6½ in., Limicolaria, Perideris, and Pseudachatina are the characteristic forms. The Agnatha are represented by Ennea, Streptaxis, and Streptostele. Rachis and Pachnodus, sub-genera of Buliminus, occur also on the east coast. A special feature is the development of several peculiar slug-like genera, e.g. Oopelta, perhaps a form of Arion; Estria, a slug with an external shell, akin to Parmacella; and Aspidelus, a form intermediate between Helicarion and Limax. Claviger, a handsome group akin to Cerithium, is peculiar to the estuaries of West African rivers.
About sixteen species are known from the Cameroons District, but no peculiar genera occur. The French Congo District has[330] not yet been well explored. Tomostele, a genus allied to Streptostele, is peculiar, and Pseudachatina attains its maximum.

Fig. 217.—Columna flammea Müll., Princes I.
St. Thomas and Princes Is., in the Gulf of Guinea, are well known. Princes I. has 22 species, 14 peculiar, and 2 common to St. Thomas only, one of the latter being the great sinistral Achatina bicarinata Chem. The remarkable genus Columna (Fig. 217) is peculiar, and Streptostele (4 sp.) attains its maximum. Peculiar to St. Thomas are Pyrgina, a turreted form of Stenogyra; Thyrophorella, a sinistral form of Zonites; and Atopocochlis, a large bulimoid shell, whose true relationships are not yet known. Homorus, a group of Achatina with an elongated spire, occurring also in the Angola District and on the east coast, has 4 species. No fresh-water species have as yet been discovered in either of the islands.
The Angola and Benguela District, extending from the Congo to the Cunene R., probably belongs to the West African Sub-region, but until its fauna is better known it is advisable to consider it apart. Achatina continues abundant, but the other characteristic West African forms (Pseudachatina, Streptostele, Perideris) diminish or are absent altogether. No Helix and only 1 Cyclophorus occur.
Ovampo, Damara, and Great Namaqualand, lying between the Cumene and Orange rivers, seem to form a transition district between the West and South African faunas. Helix reappears, while the characteristic West African genera are almost entirely wanting.
(c) The East African Province extends from about Delagoa Bay to the Abyssinian shores of the Red Sea. In general out-line the province consists of a flat marshy district, extending inland for many miles from the sea; this is succeeded by rising ground, which eventually becomes a high table-land, often desolate and arid, whose line of slope lies parallel to the trend of the coast. The Mollusca are little known, and have only been studied in isolated districts, usually from the discoveries of exploring expeditions.
The Mozambique District, from Delagoa Bay to Cape Delgado,[331] includes no genus which does not occur on the west coast, except Cyclostoma (2 sp.). Trochonanina (4 sp.), Urocyclus, a characteristic African slug (2 sp.), Rachis (6 sp.), Pachnodus (2 sp.), and Achatina (5 sp.), are the principal groups.
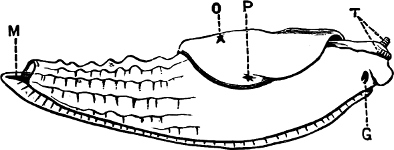
Fig. 218.—Urocyclus comorensis Fisch., Comoro Is.: G, Generative orifice; M, mucous gland; O, orifice leading to internal shell; P, pulmonary orifice; T, tentacles. (After Fischer.)
The Zanzibar District, from Cape Delgado to the Somali country, has the same general features. Meladomus, a large sinistral Ampullaria, is characteristic, while Cyclostoma (5 sp.) becomes more abundant. Helix is still absent, but the carnivorous forms (Streptaxis 2 sp., Ennea 7 sp.) are rather numerous.
The Somali District is characterised by operculate groups of the Otopoma type (Georgia, Rochebrunia, Revoilia) whose generic value is rather doubtful. Petraeus, in an Arabian type, supplants Rachis and Pachnodus. Achatina is nearly wanting, but Limicolaria has 9 species. A few Helix, said to be of the Pisana group, occur.
The District between the Great Lakes and the coast region is fairly well known through recent explorations, especially those associated with Emin Pasha. Streptaxis (6 sp.) and Ennea (24 sp.) are numerous, Helix is wanting, and the Naninidae are represented by Trochonanina (7 sp.), and other forms at present grouped under Nanina or Hyalinia. On the high ground Buliminus, Cerastus, and Hapalus replace, to some extent, the Achatina and Limicolaria of the marshy plains. Land operculates (Cyclophorus 1, Cyclostoma 8) are more numerous; among fresh-water genera we have Lanistes (5 sp.), Cleopatra (3 sp.), Meladomus (1 sp.), and Leroya, a sinistral form with the facies of a Littorina. The characteristic African bivalves (Mutela, Spatha, etc.) are few in number.
(d) Province of the Great Lakes.—The Mollusca of the four great lakes of Eastern Central Africa—Lakes Albert Nyanza (Luta Nzige, 2720 ft.), Victoria Nyanza (Oukéréwé, 3700 ft.), Nyassa (1520 ft.), and Tanganyika (2800 ft.)—are well known, and supply an interesting problem in distribution. Those of the[332] three first mentioned lakes differ in no way from the rest of tropical Africa, but the Mollusca of Tanganyika include, in addition to the ordinary African element, a number of peculiar operculate genera, belonging principally to the Melaniidae and Hydrobiidae. Several of these possess a solidity of form and compactness of structure which is unusual in fresh-water genera, and has led to the belief, among some authorities, that they are the direct descendants of marine species, and that Tanganyika represents an ancient marine area. This view appears untenable. The Victoria Nyanza and Nyassa are part of the same system as Tanganyika, and it is not easy to see how, if Tanganyika were once an arm of the sea, they were not equally so, especially as they are several hundred miles nearer the Indian Ocean as at present defined. Nor, as will be seen from the figures given above, is there anything in the altitudes which would make us expect anything exceptional in Tanganyika. The similar case of L. Baikal must be compared (p. 290), where again a number of specialised forms of Hydrobia occur.
Of the genera concerned, Paramelania and Nassopsis are forms of Melaniidae; Tiphobia (Fig. 219), which is allied to Paludomus, is a compact shell with angulated spinose whorls; Lacunopsis, Ponsonbya, Limnotrochus, and Tanganyicia are probably forms of Lithoglyphus, some, as their names denote, being of decidedly marine facies; Syrnolopsis and Turbonilla (?) look like Pyramidellidae, Horea and Reymondia like Rissoina; Bourguignatia appears to belong to Vivipara, with which has now been merged the genus Neothauma. Recently discovered forms from the adjacent L. Mweru are evidently of kindred origin.
(e) The Afro-Arabian Province includes Abyssinia, with S. Arabia, the African shores of the Gulf of Aden, and Socotra. The province contains a singular mixture of types. The high ground of Abyssinia stands like a lofty European island in the midst of a tropical plain, with Palaearctic genera flourishing like hardy northern plants on a mountain in low latitudes. Helix, Vitrina, and Pupa abound, with a few Clausilia and even a Limax. On the lower levels occur Limicolaria (3 sp.), Subulina (7 sp.), Helicarion, and Homorus, but land operculates are entirely wanting. Characteristic of the province as a whole are various forms of Buliminus, which in Socotra are represented by two peculiar sub-genera, Achatinelloides and Passamaiella. In S.[333] Arabia the mixture of types produces curious results: the Helix, Clausilia, and Vitrina being Palaearctic, the Limicolaria and all the operculates Ethiopian, while the single Trochomorpha is Indian. Indian influence, indeed, comes out unmistakably throughout the province. Thus in Socotra there are two Cyclotopsis, in Abyssinia two Africarion (closely related to the Indian Girasia), two Microcystis, and a Glessula, and in the Scioa district there is a Sitala. The fresh-water Mollusca of Socotra are Indian forms.
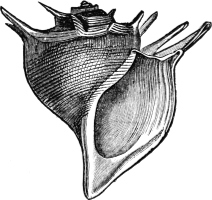
Fig. 219.—Tiphobia Horei E. A. Smith, L. Tanganyika.
Fig. 220.—Mollusca characteristic of L. Tanganyika: A, Nassopsis nassa Woodw.; B, Spekia zonata Woodw.; C, Syrnolopsis lacustris E. A. Smith.

Fig. 221.—Achatina zebra Lam., S. Africa. × ½.
(2) The South African Sub-region.—The principal characteristic of the Mollusca of S. Africa is the occurrence of numerous small species of Helicidae, belonging chiefly to the groups Pella, Phasis, Dorcasia, and Sculptaria, all of which are practically peculiar. Carnivorous genera are also prominent, Ennea here attaining its maximum. Rhytida (to which several species still regarded as Pella belong) is common only to the S. Pacific and Australasia, and forms, with Isidora among the fresh-water pulmonates, a remarkable link of connexion. Aerope, the largest of all helicoid carnivorous genera, and Chlamydephorus, a carnivorous slug with an internal shell, are peculiar. Achatina is still abundant, but Limicolaria is wanting. Livinhacea, a[334] form with a continuous peristome, perhaps akin to Bulimus; Apera, a form of slug; and Coeliaxis, a genus perhaps akin to the Papuan and Queensland Perrieria, are all peculiar. The land operculates, which are not numerous, are of the East African type.
Land Mollusca of the S. African Sub-region
| Chlamydephorus | 1 |
| Ennea | 31 |
| Aerope | 5 |
| Rhytida | 3 |
| Helicarion | 3 |
| Trochonanina | 1 |
| Trochozonites | 1 |
| Limax | 1 |
| Apera | 1 |
| Vitrina | 7 |
| Nanina | 6 |
| Conulus | 2 |
| Patula | 2 |
| Pella | 44 |
| Dorcasia | 8 |
| Phasis | 1 |
| Sculptaria | 2 |
| Helix (inc. sed.) | 4 |
| Rachis | 1 |
| Pachnodus | 3 |
| Buliminus (?) | 4 |
| Pupa | 20 |
| Vertigo | 2 |
| Achatina | 18 |
| Livinhacea | 1 |
| Stenogyra | 4 |
| Coeliaxis | 1 |
| Succinea | 3 |
| Vaginula | 2 |
| Cyclophorus | 1 |
| Cyclostoma | 7 |
| Cyclotus (?) | 1 |
| Blanfordia | 1 |
St. Helena.—The Molluscan fauna of St. Helena is perhaps the most puzzling, as regards its geographical affinities, of any in the world. It consists of 29 peculiar species of land Mollusca (fresh-water species being unknown), 19 of which are recently extinct, partly owing to the destruction of the forest, but are found in considerable abundance in a state of good preservation.[375] The genera are—
| Hyalinia | 1 |
| Patula | 4 (3 extinct) |
| Endodonta | 10 (7 extinct) |
| Bulimulus | 7 (5 extinct) |
| Pachyotus | 1 (extinct) |
| Tomigerus (?) | 1 (extinct) |
| Pupa | 2 (extinct) |
| Succinea | 3 |
The 5 genera which concentrate our attention are Patula, Endodonta, Pachyotus (Fig. 222), Tomigerus, and Bulimulus, all of which appear utterly strange to an oceanic island in the middle of the S. Atlantic. Patula and Endodonta are essentially Polynesian forms, occurring abundantly on all the island groups in the Central Pacific. Pachyotus, Tomigerus (assuming its correct identification), and Bulimulus are all S. American forms, the two former being especially characteristic of Brazil. How this mixture of genera now confined to regions so widely distant, not only from St. Helena itself, but from one another, became associated here, is a problem obviously not easy of solution. The fauna is probably a remnant of a very ancient type, possibly at one[335] time much more widely distributed. Endodonta (an essentially insular form, like Omphalotropis) actually occurs on Fernando Noronha, off the Brazil coast, and we shall see how an Indian and even a Polynesian element is present off the eastern coasts of Africa.
Ascension I.—One indigenous species, a so-called Limax, is all that has ever been discovered.
(3) The Malagasy Sub-region includes Madagascar with its attendant satellites Bourbon, Mauritius, and Rodriguez, and the Seychelles and Comoro groups. No land Mollusca are known from the Amirantes, the Chagos, or from Aldabra. The special characteristics of the sub-region are the great development of the carnivorous land Mollusca (Ennea, Gibbus), the occurrence of a considerable number of true Helicidae of great size and beauty, and the prominence of the genus Cyclostoma.
(a) The Madagascan Province.—The land Mollusca of Madagascar, although as yet imperfectly known, possess a striking individuality. Two of the chief characteristics of the Ethiopian region are the paucity of its land operculate and of its Helix fauna; Madagascar is especially distinguished by the rich development of both these groups. For size, colouring, and beauty of shape, the Helicidae of the two sub-genera Ampelita and Helicophanta rival, if they do not surpass, any in the world. They are quite peculiar to this sub-region, not a trace of them occurring on the Mascarenes, Seychelles, or even on the Comoros. Helicophanta is distinguished by the enormous size of its embryonic shell, which persists in the adult (Fig. 223), and in this respect the group appears to be related to Acavus (Ceylon, Fig. 204) and Panda (N.E. Australia). As is usual when Helix is well developed, Nanina (about 12 sp.) is proportionately scanty.
The African Bulimini (Pachnodus and Rachis) are represented by two species, but Achatina, so abundant on the mainland, is scarce. Two other groups of Buliminus, Leucotaenia and Clavator, are peculiar. The presence of a single Kaliella, specifically identical with a common Indian form, is very remarkable.
Cyclostoma proper, of which Madagascar is the metropolis, is richly developed (54 sp.). Many of the species are of great size and of striking beauty of ornamentation. Unlike its Helicidae, this genus is not restricted to Madagascar; several species[336] occur on the mainland, 6 on the Comoros, one on the Seychelles, and 16 in Mauritius. The sub-genera Acroptychia and Hainesia are peculiar.

Fig. 222.—Pachyotus auris vulpina Desh., St. Helena (sub-fossil).
Fig. 223.—Helix (Helicophanta) Souverbiana Fisch., Madagascar, showing embryonic shell. × ⅔.
Fig. 224.—Cyclostoma campanulatum Pfr., Madagascar.
The fresh-water Mollusca of Madagascar contain further traces of Indian relationship. Thus we find two species of Paludomus, a genus whose metropolis is Ceylon, India, and Further India, and which is barely represented on the Seychelles and in the Somali district. Melanatria, which is peculiar to Madagascar, has its nearest affinities in the Cingalese and East Indian faunas. Several of the Melania and the two Bithynia are of a type entirely wanting in Africa, but common in the Indo-Malay sub-region. Not a single one of the characteristic African fresh-water bivalves (Mutela, Spatha, Aetheria, Galatea, etc.) has been found in Madagascar. On the other hand, certain African Gasteropoda, such as Cleopatra and Isidora, occur, indicating, in common with the land Mollusca, that an ultimate land connexion with Africa must have taken place, but at an immeasurably remote period.
[337]
Land and Fresh-water Mollusca of Madagascar
| Ennea | 9 |
| Urocyclus | 2 |
| Helicarion (?) | 1 |
| Macrocyclis (?) | 1 |
| Kaliella | 1 |
| Nanina (inc. sed.) | 9 |
| Ampelita | 35 |
| Helicophanta | 17 |
| Pachnodus | 2 |
| Rachis | 2 |
| Leucotaenia | 2 |
| Clavator | 2 |
| Achatina | 3 |
| Opeas | 2 |
| Subulina | 3 |
| Vaginula | 4 |
| Limnea | 2 |
| Planorbis | 3 |
| Isidora | 3 |
| Melania | 7 |
| Melanatria | 4 |
| Paludomus | 2 |
| Vivipara | 1 |
| Bithynia | 2 |
| Cleopatra | 2 |
| Ampullaria | 6 |
| Cyclophorus | 2 |
| Cyclotus (?) | 1 |
| Cyclostoma | 54 |
| Otopoma | 5 |
| Lithidion | 1 |
| Acroptychia | 3 |
| Hainesia | 3 |
| Unio | 1 |
| Corbicula | 2 |
| Sphaerium | 1 |
| Pisidium | 1 |
The Comoro Islands.—This isolated group possesses about 100 species, almost all of which are peculiar. The principal feature is the rich development of Ennea (30 sp.). On the whole the group shows more relationship to Madagascar than to the mainland. Thus we have six species of true Cyclostoma, and only one Achatina, while among the fresh-water genera is Septaria, which is characteristic of the whole Malagasy Sub-region, but is absent from the mainland. The Helicidae are all of insignificant size. Peculiar to the group is the remarkable genus Cyclosurus (Fig. 152, p. 247).
(b) The Mascarene Province (Mauritius, Bourbon, Rodriguez, and the Seychelles).—The percentage of peculiar species, which is very high, can only be paralleled in the case of some of the West Indian islands, and sufficiently attests the extreme isolation of the group from Madagascar. We have—
| Total sp. | Land sp. | Fresh-water sp. |
Peculiar | Peculiar to group |
|
| Mauritius | 113 | 104 | 9 | 78 | 102 (90 p.c.) |
| Bourbon | 45 | 40 | 5 | 19 | 38 (84 p.c.) |
| Rodriguez | 23 | 19 | 4 | 15 | 21 (95 p.c.) |
| Seychelles | 34 | 27 | 7 | 24 | 30 (90 p.c.) |
The Mollusca of the group exhibit three distinct elements, the Indigenous, the Madagascan, and the Indian and Australasian.
The genus Pachystyla (Naninidae) is quite peculiar, forming the main portion of the land snails proper. It attains its maximum in Mauritius (17 sp.), with 5 sp. in Bourbon and one sub-fossil sp. in Rodriguez, while in the Seychelles it is absent. But the principal feature of the Mascarene group is the extraordinary development of the carnivorous genus Gibbus, which[338] has 27 sp. in Mauritius, 8 in Bourbon, 4 in Rodriguez; in the Seychelles, it is replaced by Edentulina and Streptostele. The principal link with Madagascar is found in a part of the operculate land fauna. Cyclostoma is present (with Otopoma) in several fine living forms, and the number of sub-fossil species is a clear indication that this group was, not long ago, much more abundant, for of the 16 Cyclostoma known from Mauritius 10 are sub-fossil. The operculates form a decided feature of the land fauna; thus in Mauritius there are 32 species, or more than 28 per cent of the whole.
Fig. 225.—Characteristic Mauritian land shells: A, Gibbus palanga Fér.; A´, young of same; B, Gibbus lyonetianus Pall.
Indian and Australasian affinities are unmistakably present. Thus Omphalotropis, a genus characteristic of small islands, is profusely represented, but it does not occur in Madagascar or Africa. Two Helicina (Mauritius and Seychelles) and a single Leptopoma (possibly a Leptopomoides) are also of eastern relationship. Cyclotopsis, Cyathopoma, and Geostilbia are markedly Indian genera. Microcystis, Patula, and Tornatellina are Polynesian. Hyalimax—and this is a very striking fact—occurs nowhere else but in the Andamans and Nicobars, and on the Aracan coast. The nearest relation to the Seychelles Mariaella appears to be the Cingalese Tennentia. Not a single representative of these eleven genera has been found even in Madagascar.
The fresh-water Mollusca (omitting the Neritidae) are: Mauritius 9 species, Bourbon 5, Rodriguez 4, Seychelles 6, with only 15 species in all. The one Planorbis and the Vivipara, the Paludomus and two of the Melania are of Indian types. The Lantzia (peculiar to Bourbon) is probably allied to the Indian Camptonyx. Owing to the paucity of permanent streams, no fresh-water bivalves occur. Among the Neritidae is a single Septaria, a genus which, though occurring in Madagascar, is entirely strange to Africa, and is abundant in the Oriental and Australasian regions.
It would seem probable that when the closer connexion which[339] at one time undoubtedly existed between India and Eastern Africa began to be less continuous,[376] the Mascarene group was first severed from what ultimately became Madagascar, while the Seychelles, and perhaps the Comoros, still continued united to it. The Comoros, which lack the great Helices, separated off from Madagascar first, while the Seychelles continued in more or less direct union with that island sufficiently long to receive the progenitors of Stylodonta (a peculiar group of Helix), but became disunited at an exceedingly remote period.
The southern boundary of this region may be regarded as roughly corresponding to that of the United States, i.e. Lower California and Mexico are excluded. The southern portion of Florida belongs to the Antillean sub-region.
The principal characteristic of the Nearctic Region is the remarkable poverty of its land Mollusca. No district in the world of equal extent is so poor in genera, while those which occur are generally of small size, with scarcely anything remarkable either in colouring or form. The elongated land shells (Clausilia, Buliminus), so characteristic of Europe, are entirely wanting, but a few Bulimulus, of Neotropical origin, penetrate Texas, and from the same sources come a few species of Glandina (as far north as S. Carolina), Holospira (Texas), and Helicina.
The region falls into two well-marked sub-regions, the N. American and the Californian, with the Rocky Mountain district as a sort of debatable ground between them. The Californian sub-region consists of the narrow strip of country between the Sierra Nevada, the Cascade Mountains and the coast-line, from San Diego to Alaska; the N. American sub-region consists of the remainder of the region.
(1) The N. American Sub-region.—The Carnivorous genera are represented solely by the few Glandina mentioned above, and by the indigenous genus Selenites, a form midway between Testacella and Limax, whose metropolis is on the Pacific slope,[340] but which spreads eastward into the Antilles. Among the Limacidae, Limax is common to both sub-regions, but Tebennophorus (4 sp., 3 of which belong to the genus Pallifera), a genus found also in China and Siam, and Vitrinozonites do not occur in the Californian. Hyalinia (Zonites) is fairly abundant, especially in the groups Mesomphix and Gastrodonta (peculiar to this sub-region), and Hyalinia proper. Patula is well represented. The Helicidae belong principally to the groups Mesodon, Stenotrema, Triodopsis, Polygyra, and Strobila, only 6 of which, out of a total of 84, reach the Pacific slope. Land operculates are conspicuous for their almost complete absence (see map, frontispiece).
Fig. 226.—Characteristic North American Mollusca. A, Helix (Mesodon) palliata Say, Ohio. B, Helix (Polygyra) cereolus Mühlf., Texas. C, Patula alternata Say, Tennessee.
The poverty of the land fauna is atoned for by the extraordinary abundance and variety of the fresh-water genera. A family of operculates, the Pleuroceridae, with 10 genera and about 450 species, is quite peculiar, a few stragglers only reaching Central America and the Antilles. The nucleus of their distribution is the Upper Tennessee River with its branches, and the Coosa River. They appear to dislike the neighbourhood of the sea, and are never found numerously within 100 miles of it. They adhere to stones in rapid water, and differ from the Melaniidae of the Old World and of S. America in the absence of a fringe to the mantle and in being oviparous. They do not occur north of the St. Lawrence River, or north of U.S. territory in the west, or in New England. Three-quarters of all the known species inhabit the rough square formed by the Tennessee River, the Mississippi, the Chattahoochee River, and the Gulf of Mexico. The Mississippi is a formidable barrier to their extension, and a whole section (Trypanostoma, with the four genera Io, Pleurocera, Angitrema, and Lithasia) does not occur west of that river. The Viviparidae are also very largely developed, the genera Melantho, Lioplax, and Tulotoma being peculiar. The Pulmonata are also abundant, while the richness[341] of the Unionidae may be gathered from the fact that Wetherby states[377] that in 1874 no less than 832 species in all had been described.
The entire Mississippi basin is inhabited by a common assemblage of Unionidae, and a considerable number of the species are distributed over the whole of this area, Texas, and parts of E. Mexico. Some species have spread out of this area into Michigan, Canada, the Red River, and Hudson’s Bay district, and even into streams in New York which drain into the Atlantic. An entirely different set of forms occupy the great majority of the rivers falling into the Atlantic, the Appalachian Mountains acting as an effective barrier between the two groups of species, which appear to mingle below the southern end of the range. In many cases Unionidae seem to have no difficulty in migrating from river to river, if the distance is not extreme; they probably are carried across overflowed districts in time of flood.[378]

Fig. 227.—Helix (Arionta) fidelis Gray, Oregon.
(2) The Californian Sub-region is markedly distinct from the rest of N. America. The characteristic sombre Helices of the Eastern States are almost entirely wanting, and are replaced by Arionta (20 sp.), a larger and more varied group, which may have some affinity to Chinese forms. Glyptostoma (1 sp.) is also peculiar. Selenites here has its metropolis, and Pristiolma is a remarkable group of small Hyalinia (Zonites), but the larger forms of the Eastern States are wanting. Several remarkable and quite peculiar forms of slug occur, namely, Ariolimax (whose nearest relation is Arion), Prophysaon, Hemphillia, and Binneya. There are no land operculates.
Not more than 15 to 20 species of the Pleuroceridae (sect. Goniobasis) occur west of the Rocky Mountains, and only a single Unio, 5 Anodonta, and 1 Margaritana, which is common to New England. Pompholyx is a very remarkable ultra-dextral form of Limnaea, apparently akin to the Choanomphalus of[342] L. Baikal. Bithynia, absent from the Eastern States, is represented by two species. The general indications are in favour of the Californian fauna having migrated from an Old World source after the upheaval of the Sierras; the American fauna, on the other hand, is purely indigenous, with no recent Old World influence at all.
Land Mollusca of the Nearctic Region
| Glandina | 4 |
| Selenites | 6 |
| Limax | 4 |
| Vitrina | 4 |
| Vitrinozonites | 1 |
| Mesomphix | 15 |
| Hyalinia | 22 |
| Conulus | 1 |
| Gastrodonta | 9 |
| Pristiloma | 2 |
| Tebennophorus | 4 |
| Ariolimax | 6 |
| Prophysaon | 2 |
| Hemphillia | 1 |
| Binneya | 1 |
| Patula | 18 |
| Punctum | 2 |
| Arionta | 20 |
| Praticola | 2 |
| Glyptostoma | 1 |
| Mesodon | 27 |
| Stenotrema | 11 |
| Triodopsis | 21 |
| Polygyra | 23 |
| Polygyrella | 2 |
| Gonostoma | 1 |
| Vallonia | 1 |
| Strobila | 2 |
| Pupa | 18 |
| Vertigo | 8 |
| Holospira | 2 |
| Cionella | 1 |
| Bulimulus | 6 |
| Macroceramus | 1 |
| Succinea | 21 |
| Vaginulus | 1 |
| Helicina | 2 |
The land Mollusca of the Neotropical Region stand in complete contrast to those of the Nearctic. Instead of being scanty, they are exceedingly abundant; instead of being small and obscure, they are among the largest in size, most brilliant in colour, and most singular in shape that are known to exist. At the same time they are, as a whole, isolated in type, and exhibit but little relation with the Mollusca of any other region.
The most marked feature is the predominance of the peculiar genera Bulimus and Bulimulus, the centre of whose development appears to lie in Peru, Ecuador, and Bolivia, but which diminish, both in numbers and variety of form, in the eastern portion of the region. In the forests of Central America, Venezuela, and Ecuador, and, to a lesser degree, in those of Peru and Brazil, occurs the genus Orthalicus, whose tree-climbing habits recall the Cochlostyla of the Philippines. These three groups of bulimoid forms constitute, as far as the mainland is concerned, the preponderating mass of the land Mollusca. Helix proper is most strongly developed in the Greater Antilles, which possess several peculiar groups of great beauty. In Central America Helix is comparatively scarce, but in the northern portions of the continent several fine genera (Labyrinthus,[343] Isomeria, Solaropsis) occur, which disappear altogether towards the south.
Carnivorous land Mollusca are, so far as Central America is concerned, more highly developed than in any other quarter of the world, particularly in the genera Glandina and Streptostyla. These genera also penetrate the northern portions of the continent, Glandina reaching as far as Ecuador, and Streptostyla as far as Peru. The Greater Antilles have also characteristic forms of these genera. Streptaxis is tolerably abundant all over tropical South America, and is the one pulmonate genus which shows any affinity with the African fauna.
The slugs are exceedingly scarce. Vaginula occurs throughout, and is the only genus in any sense characteristic.
Clausilia, in the sub-genus Nenia, occurs along the Andean chain from the extreme north (but not in Central America) as far south as Bolivia. It has in all probability made its way into S. America in exceedingly remote ages from its headquarters in Eastern Asia. No species survives in N. America, and a single straggler is found in Porto Rico. The genera Macroceramus, Cylindrella, and Strophia, are characteristic West Indian forms, which are only slightly represented on the mainland. Homalonyx, a curious form akin to Succinea, is peculiar to the region.
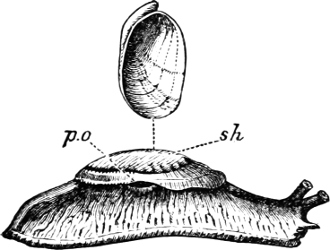
Fig. 228.—Homalonyx unguis Fér., Demerara. sh, Shell (shown also separate); p.o, pulmonary orifice.
Land operculates attain a most extraordinary development in the Greater Antilles, and constitute, in some cases, nearly one-half of the whole Molluscan fauna. Several groups of the Cyclostomatidae find their headquarters here, and some spread no farther. On the mainland this prominence does not continue. West Indian influence is felt in Central America and on the northern coast district, and some Antillean genera make their way as far as Ecuador. The whole group entirely disappears in Chili and Argentina, becoming scarce even in Brazil.
Among the fresh-water operculates, Ampullaria is abundant, and widely distributed. Vivipara, so characteristic of N. America, is entirely absent. Chilina, a remarkable fresh-water pulmonate, akin to Limnaea, is peculiar to Chili, Patagonia, and[344] Southern Brazil, but is not found in the tropical portion of the continent. Of the fresh-water Pelecypoda Mycetopus, Hyria, Castalia, Leila, and Mülleria are peculiar forms, akin to the Unionidae.
(1) The Antillean Sub-region surpasses all other districts in the world in respect of (1) extraordinary abundance of species, (2) sharp definition of limits as a whole, (3) extreme localisation of the fauna of the separate islands. The sub-region includes the whole of the half-circle of islands from the Bahamas to Grenada, together with the extreme southern end of the peninsula of Florida, which was once, no doubt, a number of small islands like the Bahamas. Trinidad, and probably Tobago, although containing an Antillean element, belong to the mainland of S. America, from which they are only separated by very shallow water.
The sub-region appears to fall into four provinces:—
(a) Cuba, the Bahamas, and S. Florida; (b) Jamaica; (c) San Domingo (Haiti), Porto Rico, and the Virgin Is., with the Anguilla and St. Bartholomew group; (d) the islands from Guadeloupe to Grenada. The first three provinces contain the mass of the characteristic Antillean fauna, the primary feature being the extraordinary development of the land operculates, which here reaches a point unsurpassed in any other quarter of the globe. The relative numbers are as follows:—
| Cuba | Jamaica | San Domingo | Porto Rico | |
| Inoperculate | 362 | 221 | 152 | 75 |
| Operculate | 252 | 242 | 100 | 23 |
It appears, then, that the proportion of operculate to inoperculate species, while very high in Cuba (about 41 per cent of the whole), reaches its maximum in Jamaica (where the operculates are actually in a majority), begins to decline in San Domingo (about 40 per cent), and continues to do so in Porto Rico, where they are not more than 24 per cent of the whole. These operculates almost all belong to the families Cyclostomatidae and Helicinidae, only two genera (Aperostoma and Megalomastoma) belonging to the Cyclophorus group. Comparatively few genera are absolutely peculiar to the islands, one or two species of most of them occurring in Central or S. America, but of the several hundreds of operculate species which occur on the islands, not two score are common to the mainland.
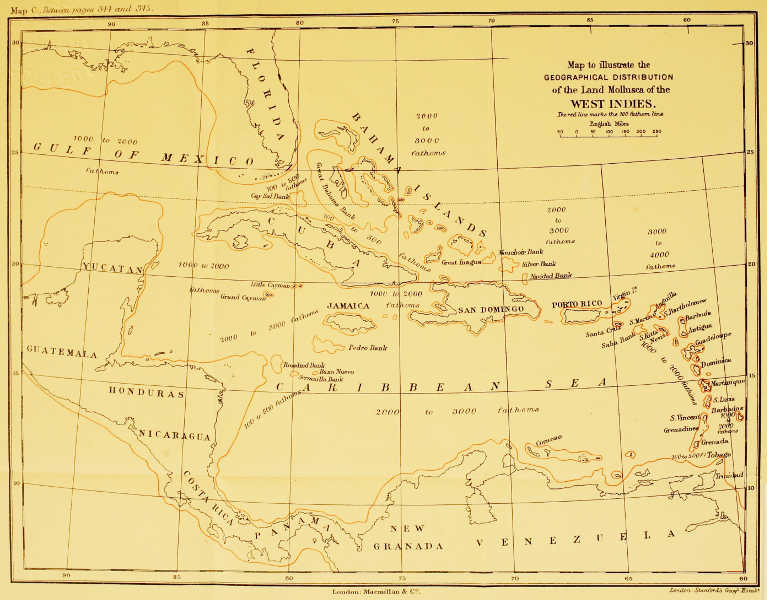
Map to illustrate the
GEOGRAPHICAL DISTRIBUTION
of the Land Mollusca of the
WEST INDIES.
The red line marks the 100 fathom line.
London: Macmillan and Cọ.
London: Stanford’s Geogḷ Estabṭ.
[345]
The next special feature of the sub-region is a remarkable development of peculiar sub-genera of Helix. In this respect the Antilles present a striking contrast to both Central and S. America, where the prime feature of the land Pulmonata is the profusion of Bulimus and Bulimulus, and Helix is relatively obscured. No less than 14 sub-genera of Helix, some of which contain species of almost unique beauty and size, are quite peculiar to the Greater Antilles, and some are peculiar to individual islands.
Here, too, is the metropolis of Cylindrella (of which there are 130 species in Cuba alone), a genus which just reaches S. America, and has a few species along the eastern sea-board of the Gulf of Mexico. Macroceramus and Strophia are quite peculiar; the former, a genus allied to Cylindrella, which attains its maximum in Cuba and San Domingo, is scarcely represented in Jamaica, and disappears south of Anguilla; the latter, a singular form, resembling a large Pupa in shape, which also attains its maximum in Cuba, is entirely wanting in Jamaica, and has its last representative in S. Croix. One species irregularly occurs at Curaçao.
The carnivorous group of land Mollusca are represented by several peculiar forms of Glandina, which attain their maximum in Jamaica and Cuba, but entirely disappear in the Lesser Antilles.
A certain number of the characteristic N. American genera are found in the Antillean Sub-region, indicating a former connexion, more or less intimate, between the W. Indies and the mainland. The genera are all of small size. The characteristic N. American Hyalinia are represented in Cuba, San Domingo, and Porto Rico; among the Helicidae, Polygyra reaches Cuba, but no farther, and Strobila Jamaica. The fresh-water Pulmonata are of a N. American type, as far as the Greater Antilles are concerned, but the occurrence of Gundlachia (Tasmania and Trinidad only) in Cuba is an unexplained problem at present. Unionidae significantly occur only at the two ends of the chain of islands, not reaching farther than Cuba (Unio 3 sp.) at one end, and Trinidad (which is S. American) at the other.
A small amount of S. American influence is perceptible throughout the Antilles, chiefly in the occurrence of a few species of Bulimulus and Simpulopsis. The S. American element may[346] have strayed into the sub-region by three distinct routes: (1) by way of Trinidad, Tobago, and the islands northward; (2) by a north-easterly extension of Honduras towards Jamaica, forming a series of islands of which the Rosalind and Pedro banks are perhaps the remains; (3) by a similar approximation of the peninsula of Yucatan and the western extremity of Cuba. Central America is essentially S. American in its fauna, and the characteristic genera of Antillean operculates which occur on its eastern coasts are sufficient evidence of the previous existence of a land connexion more or less intimate (see map).
(a) Cuba is by far the richest of the Antilles in land Mollusca, but it must be remembered that it is also much better explored than San Domingo, the only island likely to rival it in point of numbers. It contains in all 658 species, of which 620 are land and 38 fresh-water, the land operculates alone amounting to 252.
Carnivorous genera form but a small proportion of the whole. There are 18 Glandina (which belong to the sections Varicella and Boltenia) and 4 Streptostyla, the occurrence of this latter genus being peculiar to Cuba and Haiti (1 sp.) among the Antilles, and associating them closely with the mainland of Central America, where Streptostyla is abundant. These two genera alone represent the Agnatha throughout the sub-region.
There are no less than 84 species of Helix, belonging to 12 sub-genera. Only one of these (Polymita) is quite peculiar to Cuba, but of 7 known species of Jeanerettia and 8 of Coryda, 6 and 7 respectively are Cuban. Thelidomus has 15 species (Jamaica 3, Porto Rico 3); Polydontes has 3, the only other being from Porto Rico; Hemitrochus has 12 (Jamaica 1, Bahamas 6); Cysticopsis 9 (Jamaica 6); Eurycampta 4 (Bahamas 1).
The Cylindrellidae find their maximum development in Cuba. As many as 34 Macroceramus occur (two-thirds of the known species), and 130 Cylindrella, some of the latter being most remarkable in form (see Fig. 151, B, p. 247).
The land operculates belong principally to the families Cyclostomatidae and Helicinidae. Of the former, Cuba is the metropolis of Ctenopoma and Chondropoma, the former of which includes 30 Cuban species, as compared with 1 from San Domingo and 2 from Jamaica. Megalomastoma (Cyclophoridae) is also Haitian and Porto Rican, but not Jamaican. Blaesospira, Xenopoma, and Diplopoma are peculiar. The Helicinidae consist[347] mainly of Helicina proper (58 sp.), which here attains by far its finest development in point of size and beauty, and of Eutrochatella (21 sp.), which is peculiar to the three great islands (Jamaica 6 sp., San Domingo 6 sp.).
The Bahamas, consisting in all of more than 700 islands, are very imperfectly known, but appear to be related partly to Cuba, partly to San Domingo, from each of which they are separated by a narrow channel of very deep water. They are certainly not rich in the characteristic groups of the Greater Antilles. The principal forms of Helix are Plagioptycha (6 sp.), common with San Domingo, and Hemitrochus (6 sp.), common with Cuba. Strophia is exceedingly abundant, but Cylindrella, Macroceramus, and Glandina have but few species. There are a few species of Ctenopoma, Chondropoma, and Cistula, while a single Schasicheila (absent from the rest of the sub-region) forms a link with Mexico.
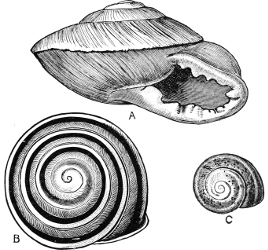
Fig. 229.—Characteristic Cuban Helices. A, Polydontes imperator Montf. B, Caracolus rostrata Pfr. C, Polymita muscarum Lea.
Southern Florida, with one or two species each of Hemitrochus, Cylindrella, Macroceramus, Strophia, Ctenopoma, and Chondropoma, belongs to this province.
(b) Jamaica.—The land Mollusca of Jamaica are, in point of numbers and variety, quite unequalled in the world. There are in all as many as 56 genera and more than 440 species, the latter being nearly all peculiar. The principal features are the[348] Glandinae, the Helicidae, and the land operculates. The Glandinae belong principally to the sub-genera Varicella, Melia, and Volutaxis, Streptostyla being absent, although occurring in Cuba and San Domingo. There are 10 genera of Helix, of which Pleurodonta is quite peculiar, while Sagda (13 sp.) is common only with S.W. San Domingo (2 sp.), and Leptoloma (8 sp.) only with Cuba (1 sp.). The single Strobila seems to be a straggler from a N. American source. Macroceramus has only 2 species as against 34 in Cuba, and of Cylindrella, in which Cuba (130 sp.) is so rich, only 36 species occur. The genus Leia, however (14 sp.), is all but peculiar, occurring elsewhere only in the neighbouring angle of San Domingo, which is so closely allied with Jamaica. The complete absence of Strophia is remarkable.
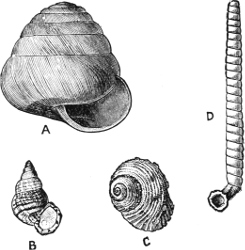
Fig. 230.—Characteristic Jamaican and Haitian Mollusca: A, Sagdae pistylium Müll., Jamaica; B, Chondropoma salleanum Pfr., San Domingo; C, Eutrochatella Tankervillei Gray, Jamaica; D, Cylindrella agnesiana C. B. Ad., Jamaica.
The land operculates form the bulk of the land fauna, there being actually 242 species, as against 221 of land Pulmonata, a proportion never again approached in any part of the world. As many as 80 of these belong to the curious little genus Stoastoma, which is all but peculiar to the island, one species having been found in San Domingo, and one in Porto Rico. Geomelania and Chittya, two singular inland forms akin to Truncatella, are quite peculiar. Alcadia reaches its maximum of 14 species, as against 4 species in San Domingo and 9 species in Cuba, and Lucidella is common to San Domingo only; but, if Stoastoma be omitted, the Helicinidae generally are not represented by so many or by so striking forms as in Cuba, which has 90 species, as against Jamaica 44, and San Domingo 35.
[349]
(c) San Domingo, although not characterised by the extraordinary richness of Cuba and Jamaica, possesses many specially remarkable forms of land Mollusca, to which a thorough exploration, when circumstances permit, will no doubt make important additions. From its geographical position, impinging as it does on all the islands of the Greater Antilles, it would be expected that the fauna of San Domingo would not exhibit equal signs of isolation, but would appear to be influenced by them severally. This is exactly what occurs, and San Domingo is consequently, although very rich in peculiar species, not equally so in peculiar genera. The south-west district shows distinct relations with Jamaica, the Jamaican genera Leia, Stoastoma, Lucidella, and the Thaumasia section of Cylindrella occurring here only. The north and north-west districts are related to Cuba, while the central district, consisting of the long band of mountainous country which traverses the island, contains the more characteristic Haitian forms.
The Helicidae are the most noteworthy of the San Domingo land Mollusca. The group Eurycratera, which contains some of the finest existing land snails, is quite peculiar, while Parthena, Cepolis, Plagioptycha, and Caracolus here reach their maximum. The Cylindrellidae are very abundant, but no section is peculiar. Land operculates do not bear quite the same proportion to the Pulmonata as in Cuba and Jamaica, but they are well represented (100 to 152); Rolleia is the only peculiar genus.
The relations of San Domingo to the neighbouring islands are considerably obscured by the fact that they are well known, while San Domingo is comparatively little explored. To this may perhaps be due the curious fact that there are actually more species common to Cuba and Porto Rico (26) than to Porto Rico and San Domingo. Cuba shares with San Domingo its small-sized Caracolus and also Liguus, but the great Eurycratera, Parthena, and Plagioptycha are wholly wanting in Cuba. The land operculates are partly related to Cuba, partly to Jamaica, thus Choanopoma, Ctenopoma, Cistula, Tudora, and many others, are represented on all these islands, while the Jamaican Stoastoma occurs on San Domingo and Porto Rico, but not on Cuba, and Lucidella is common to San Domingo and Jamaica alone. An especial link between Jamaica and San Domingo is the occurrence[350] in the south-west district of the latter island of Sagda (2 sp.). The relative numbers of the genera Strophia, Macroceramus, and Helicina, as given below (p. 351), are of interest in this connexion.
Porto Rico, with Vièque, is practically a fragment of San Domingo. The points of close relationship are the occurrence of Caracolus, Cepolis, and Parthena among the Helicidae, and of Simpulopsis, Pseudobalea, and Stoastoma. Cylindrella and Macroceramus are but poorly represented, but Strophia still occurs. The land operculates (see the Table) show equal signs of removal from the headquarters of development. Megalomastoma, however, has some striking forms. The appearance of a single Clausilia, whose nearest relations are in the northern Andes, is very remarkable. Gaeotis, which is allied to Peltella (Ecuador only), is peculiar.
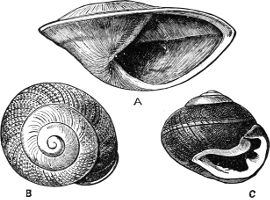
Fig. 231.—Examples of West Indian Helices: A, Helix (Parthena) angulata Fér., Porto Rico; B, Helix (Thelidomus) lima Fér., Vièque; C, Helix (Dentellaria) nux denticulata Chem., Martinique.
[351]
Land Mollusca of the Greater Antilles
| Cuba. | Jamaica. | S. Domingo. | Porto Rico. | |
| Glandina | 18 | 24 | 15 | 8 |
| Streptostyla | 4 | ... | 2 | ... |
| Volutaxis | ... | 11 (?) | 1 | ... |
| Selenites | 1 | ... | ... | ... |
| Hyalinia | 4 | 11 | 5 | 6 |
| Patula | 5 | 1 | ... | ... |
| Sagda | ... | 13 | 2 | ... |
| Microphysa | 7 | 18 | 8 | 3 |
| Cysticopsis | 9 | 6 | ... | ... |
| Hygromia (?) | ... | ... | 3 | ... |
| Leptaxis (?) | ... | ... | 1 | ... |
| Polygyra | 2 | ... | ... | ... |
| Jeanerettia | 6 | ... | ... | 1 |
| Euclasta | ... | ... | ... | 4 |
| Plagioptycha | ... | ... | 14 | 2 |
| Strobila | ... | 1 | ... | ... |
| Dialeuca | ... | 1 | ... | ... |
| Leptoloma | 1 | 8 | ... | ... |
| Eurycampta | 4 | ... | ... | ... |
| Coryda | 7 | ... | ... | ... |
| Thelidomus | 15 | 3 | ... | 3 |
| Eurycratera | ... | ... | 7 | ... |
| Parthena | ... | ... | 2 | 2 |
| Cepolis | ... | ... | 3 | 1 |
| Caracolus | 8 | ... | 6 | 2 |
| Polydontes | 3 | ... | ... | 1 |
| Hemitrochus | 12 | 1 | ... | ... |
| Polymita | 5 | ... | ... | ... |
| Pleurodonta | ... | 34 | ... | ... |
| Inc. sed. | 5 | ... | ... | ... |
| Simpulopsis | ... | ... | 1 | 1 |
| Bulimulus | 3 | 3 | 6 | 7 |
| Orthalicus | 1 | 1 | ... | ... |
| Liguus | 3 | ... | 1 | ... |
| Gaeotis | ... | ... | ... | 3 |
| Pineria | 2 | ... | ... | 1 |
| Macroceramus | 34 | 2 | 14 | 3 |
| Leia | ... | 14 | 2 | ... |
| Cylindrella | 130 | 36 | 35 | 3 |
| Pseudobalea | 2 | ... | 1 | 1 |
| Stenogyra | 6 | 7 | (?) | ... |
| Opeas | 8 | (?) | 4 | 6 |
| Subulima | 6 | 14 | 2 | 2 |
| Glandinella | 1 | ... | ... | ... |
| Spiraxis | 2 | (?) | 2 | 1 |
| Melaniella | 7 | ... | ... | ... |
| Geostilbia | 1 | ... | 1 | ... |
| Cionella | 2 | ... | ... | ... |
| Leptinaria | ... | 1 | ... | 3 |
| Obeliscus | ... | ... | 1 | 2 |
| Pupa | 2 | 7 | 3 | 2 |
| Vertigo | 4 | ... | ... | ... |
| Strophia | 19 | ... | 3 | 2 |
| Clausilia | ... | ... | ... | 1 |
| Succinea | 11 | 2 | 5 | 3 |
| Vaginula | 2 | 2 | 2 | 1 |
| Megalomastoma | 13 | ... | 1 | 3 |
| Neocyclotus | 1 | 33(?) | ... | ... |
| Licina | 1 | ... | 3 | ... |
| Jamaicia | ... | 2 | ... | ... |
| Crocidopoma | ... | 1 | 3 | ... |
| Rolleia | ... | ... | 1 | ... |
| Choanopoma | 25 | 12 | 19 | 3 |
| Ctenopoma | 30 | 2 | 1 | ... |
| Cistula | 15 | 3 | 3 | 3 |
| Chondropoma | 57 | (?) | 19 | 4 |
| Tudora | 7 | 17 | 5 | ... |
| Adamsiella | 1 | 12 | ... | ... |
| Blaesospira | 1 | ... | ... | ... |
| Xenopoma | 1 | ... | ... | ... |
| Cistula | 15 | 3 | 3 | ... |
| Colobostylus | 4 | 13 | 5 | ... |
| Diplopoma | 1 | ... | ... | ... |
| Geomelania | ... | 21 | ... | ... |
| Chittya | ... | 1 | ... | ... |
| Blandiella | ... | ... | 1 | ... |
| Stoastoma | ... | 80 | 1 | 1 |
| Eutrochatella | 21 | 6 | 6 | ... |
| Lucidella | ... | 4 | 1 | ... |
| Alcadia | 9 | 14 | 4 | ... |
| Helicina | 58 | 16 | 24 | 9 |
| Proserpina | 2 | 4 | ... | ... |
The Virgin Is., with St. Croix, Anguilla, and the St. Bartholomew group (all of which are non-volcanic islands), are related to Porto Rico, while Guadeloupe and all the islands to the south, up to Grenada (all of which are volcanic), show marked traces of S. American influence. St. Kitt’s, Antigua, and Montserrat may be regarded as intermediate between the two groups. St. Thomas, St. John, and Tortola have each one[352] Plagioptycha and one Thelidomus, while St. Croix has two sub-fossil Caracolus which are now living in Porto Rico, together with one Plagioptycha and one Thelidomus (sub-fossil). The gradual disappearance of some of the characteristic greater Antillean forms, and the appearance of S. American forms in the Lesser Antilles, is shown by the following table:—
| S | ||||||||||||||||
| P | S | S | G | M | t | |||||||||||
| o | t | S | t | u | a | S | . | |||||||||
| r | . | t | A | . | a | D | r | t | B | T | ||||||
| t | S | . | T | n | A | d | o | t | . | a | V | G | r | |||
| o | T | t | o | g | K | n | e | m | i | r | i | r | i | |||
| h | . | C | r | u | i | t | l | i | n | L | b | n | e | n | ||
| R | o | r | t | i | t | i | o | n | i | u | a | c | n | i | ||
| i | m | J | o | o | l | t | g | u | i | q | c | d | e | a | d | |
| c | a | a | i | l | l | ’ | u | p | c | u | i | o | n | d | a | |
| o | s | n | x | a | a | s | a | e | a | e | a | s | t | a | d | |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | |
| Bulimulus | 7 | 4 | 2 | 4 | 1 | 2 | 2 | 3 | 8 | 9 | 5 | 3 | 3 | 6 | 2 | 4 |
| Cylindrella | 3 | 2 | 1 | 1 | 1 | . | . | . | . | 1 | 1 | 1 | 1 | . | . | 1 |
| Macroceramus | 3 | 1 | 1 | . | 2 | 1 | . | . | . | . | . | . | . | . | . | . |
| Cyclostomatidae, etc. | 23 | 4 | 1 | 5 | 1 | 1 | 1 | . | 4 | . | . | . | . | . | . | 1 |
| Dentellaria | . | . | . | . | . | . | 1 | 1 | 8 | 5 | 11 | 2 | 2 | . | 1 | 1 |
| Cyclophorus | . | . | . | . | . | . | . | . | 1 | 2 | 2 | . | . | . | . | . |
| Amphibulimus | . | . | . | . | . | . | . | . | 2 | 3 | 1 | . | . | . | . | . |
| Homalonyx | . | . | . | . | . | . | . | . | 1 | 1 | . | . | . | . | . | . |
(d) In Guadeloupe we find Cyclophorus, Amphibulimus, Homalonyx, and Pellicula, which are characteristic of S. America, and nearly all recur in Dominica and Martinique. These islands are the metropolis of Dentellaria, a group of Helix, evidently related to some of the forms developed in the Greater Antilles. Stragglers occur as far north as St. Kitt’s and Antigua, and there are several on the mainland as far south as Cayenne. Traces of the great Bulimus, so characteristic of South America, occur as far north as S. Lucia, where also is found a Parthena (San Domingo and Porto Rico). Trinidad is markedly S. American; 55 species in all are known, of which 22 are peculiar, 28 are common to S. America (8 of these reach no farther north along the islands), and only 5 are common to the Antilles, but not to S. America. The occurrence of Gundlachia in Trinidad has already been mentioned.
The Bermudas show no very marked relationship either to the N. American or to the West Indian fauna. In common with the former they possess a Polygyra, with the latter (introduced species being excluded) one species each of Hyalosagda, Subulina, Vaginula, and Helicina, so that, on the whole, they may be called West Indian. The only peculiar group is Poecilozonites, a rather large and depressed shell of the Hyalinia type.
(2) The Central American Sub-region may be regarded as[353] extending from the political boundary of Mexico in the north to the isthmus of Panama in the south. It thus impinges on three important districts—the N. American, West Indian, and S. American; and it appears, as we should perhaps expect, that the two latter of these regions have considerably more influence upon its fauna than the former. Of the N. American Helicidae, Polygyra is abundant in Mexico only, and two species of Strobila reach N. Guatemala, while the Californian Arionta occurs in Mexico. S. American Helicidae, in the sub-genera Solaropsis and Labyrinthus, occur no farther north than Costa Rica. Not a single representative of any of the characteristic West Indian Helicidae occurs. Bulimulus and Otostomus, which form so large a proportion of the Mollusca of Venezuela, Colombia, Ecuador, and Peru, together with Orthalicus, are abundant all over the region. Again, Cylindrella, Macroceramus, and some of the characteristic Antillean operculates, are represented, their occurrence being in most cases limited to the eastern coast-line and eastern slope of the central range.
Besides these external elements, the region is rich in indigenous genera. Central America is remarkable for an immense number of large carnivorous Mollusca possessing shells. There are 49 species of Glandina, the bulk of which occur in eastern and southern Mexico; 36 of Streptostyla (S.E. Mexico and Guatemala, only 1 species reaching Venezuela and another Peru); 5 of Salasiella, 2 of Petenia, and 1 of Strebelia; the last three genera being peculiar. Streptaxis, fairly common in S. America, does not occur. Velifera and Cryptostracon, two remarkable slug-like forms, each with a single species, are peculiar to Costa Rica. Among the especial peculiarities of the region are the giant forms belonging to the Cylindrellidae, which are known as Holospira, Eucalodium, and Coelocentrum (Fig. 232). They are almost entirely peculiar to Mexico, only 7 out of a total of 33 reaching south of that district, and only 1 not occurring in it at all.
Fig. 232.—Examples of characteristic Mexican Mollusca: A, Coelocentrum turris Pfr.; B, Streptostyla Delattrei Pfr.
[354]
The land operculates are but scanty. Tomocyclus and Amphicyclotus are peculiar, and Schasicheila, a form of Helicina, occurs elsewhere only in the Bahamas. Ceres (see Fig. 18, C, p. 21) and Proserpinella, two remarkable forms of non-operculate Helicinidae (compare the Chinese Heudeia), are quite peculiar. Pachychilus, one of the characteristic fresh-water genera, belongs to the S. American (Melaniidae) type, not to the N. American (Pleuroceridae). Among the fresh-water Pulmonata, the Aplecta are remarkable for their great size and beauty. In the accompanying table “Mexico” is to be taken as including the region from the United States border up to and including the isthmus of Tehuantepec, and “Central America” as the whole region south of that point.
Land Mollusca of Central America
| Mexico only. |
Central America only. |
Common to both. |
|
| Strebelia | 1 | ... | ... |
| Glandina | 33 | 13 | 3 |
| Salasiella | 4 | ... | 1 |
| Streptostyla | 18 | 12 | 6 |
| Petenia | ... | 1 | 1 |
| Limax | ... | 1 | ... |
| Velifera | ... | 1 | ... |
| Omphalina | 10 | 1 | 1 |
| Hyalinia | 2 | 5 | 3 |
| Guppya | ... | 8 | 3 |
| Pseudohyalina | 2 | ... | 2 |
| Tebennophorus | 1 | ... | ... |
| Cryptostracon | ... | 1 | ... |
| Xanthonyx | 4 | ... | ... |
| Patula | 3 | ... | 4 |
| Acanthinula | 1 | 2 | 2 |
| Vallonia | ... | 1 | ... |
| Trichodiscus | 2 | 2 | 3 |
| Praticolella | 1 | ... | 1 |
| Arionta | 3 | ... | ... |
| Lysinoe | 1 | 1 | 1 |
| Oxychona | 2 | 5 | ... |
| Solaropsis | ... | 2 | ... |
| Polygyra | 14 | 1 | 2 |
| Strobila | 1 | 1 | ... |
| Labyrinthus | ... | 5 | ... |
| Otostomus | 23 | 20 | 7 |
| Bulimulus | 6 | 5 | 2 |
| Berendtia | 1 | ... | ... |
| Orthalicus | 6 | 3 | 3 |
| Pupa | 1 | 1 | 1 |
| Vertigo | 1 | ... | ... |
| Holospira | 12 | ... | ... |
| Coelocentrum | 6 | 1 | 1 |
| Eucalodium | 15 | ... | 5 |
| Cylindrella | 6 | 4 | ... |
| Macroceramus | 2 | 1 | ... |
| Simpulopsis | 2 | 1 | ... |
| Caecilianella | 1 | ... | ... |
| Opeas | 1 | 2 | 3 |
| Spiraxis | 8 | 2 | 1 |
| Leptinaria | ... | 2 | ... |
| Subulina | 2 | 3 | 4 |
| Succinea | 11 | 3 | 1 |
| Vaginula | 1 | ... | ... |
| Aperostoma | ... | 4 | 1 |
| Amphicyclotus | 2 | 1 | 2 |
| Cystopoma | 2 | ... | ... |
| Tomocyclus | ... | 1 | 2 |
| Choanopoma | 2 | 2 | ... |
| Chondropoma | 2 | 11 | ... |
| Helicina | 13 | 10 | 6 |
| Schasicheila | 2 | ... | 1 |
| Ceres | 2 | ... | ... |
| Proserpinella | 1 | ... | ... |
(3) The Colombian Sub-region includes Colombia, New Grenada, Venezuela, Guiana, Ecuador, Peru, and Bolivia. It has[355] been usual to separate off the two latter countries as forming a distinct “Peruvian” sub-region; but there is, as will be seen, absolutely no line to be drawn between the Mollusca of Peru and those of Ecuador; nor would one, on geographical considerations, expect to be able to draw such a line. A better method of subdivision, so far as the species of the whole eastern portion of the region are concerned, would be to group the Mollusca according to the altitude at which they occur, were it not that the evidence on this point is at present but fragmentary. We know, however, that all along the line of the Andes certain species, more particularly of Bulimulus, occupy their own zones of elevation, some ascending as high as 10,000 feet above the sea, and never occurring on the plains.
In the northern portions of this sub-region, Central American and West Indian influence is felt to a certain extent. Thus there are eight Glandina and one Streptostyla in Venezuela and Colombia together with one or two species of Cistula, Chondropoma, Proserpina, and Cylindrella, while a single Strophia (decidedly a straggler) occurs at Curaçao. In Demerara and Cayenne there are three or four species of Dentellaria. In Ecuador, however, Glandina diminishes to three species, and in Peru disappears altogether, although one Streptostyla occurs. Similarly the West Indian operculates are reduced to one Chondropoma (Ecuador) and disappear entirely in Peru.
Fig. 233.—A, Orthalicus Deburghiae Reeve, Ecuador; B, Bulimus (Pachyotus) egregius Jay, Brazil.
[356]
The Helicidae are most abundant in the north and west, and are represented by several very striking sub-genera, some of which possess remarkably toothed apertures, and perhaps betray an ancestry common to some of the West Indian genera. Of these, Labyrinthus has 12 species in Venezuela and Colombia, 5 in Ecuador, and 3 in Peru and Bolivia; Isomeria 12 in Venezuela and Colombia, 20 in Ecuador, and 2 in Peru and Bolivia; Salaropsis is represented in these countries by 6, 3, and 7 species, and Systrophia by 4, 5, and 8 species respectively.
Clausilia—in the group Nenia—appears in some numbers along the Andes chain, the only other representative in the New World being the solitary species occurring at Porto Rico. There have been described, from Venezuela and Colombia 10 species, from Ecuador 5, and from Peru and Bolivia 12.
Another marked feature of the region is the occurrence of the Orthalicidae, in the two genera Orthalicus and Porphyrobaphe. The latter of these magnificent forms is peculiar, while the former reaches Mexico, the West Indies, and Brazil. Ecuador, which contains 23 species, seems the metropolis of the group.
Bulimus and Bulimulus, the former genus being peculiar to S. America and the adjacent islands, are largely represented, the former in the three groups Borus, Dryptus, and Orphnus. These attain their maximum in Peru, with 25 species, but Venezuela and Colombia have as many as 17. Bulimulus has been subdivided into a number of groups, e.g. Drymaeus, Mesembrinus, Thaumastus, Mormus, Scutalus, with many others,—the exact scientific limits of which are not easily discernible. It must suffice here to state that Peru seems to be the headquarters of the group with about 190 species (which probably may well be reduced), Ecuador having about 70, and Venezuela and Colombia between 80 and 90.

Fig. 234.—Rhodea gigantea Mouss., New Grenada.
Two very remarkable forms belonging to the Pupidae, Anostoma (Fig. 154, p. 248) and Tomigerus, occur in Venezuela, the metropolis. Rhodea, another very peculiar shell (Fig. 234), whose exact family[357] position is uncertain, is peculiar to New Grenada. The land operculates are few in number, and in Bolivia almost disappear. They belong principally to Neocyclotus (of which 11 species occur in Venezuela and Colombia) and Helicina (10 species in the same district), besides the stragglers already mentioned from West Indian sources, and a few Cyclophorus. Bourcieria is a form of Helicina peculiar to Ecuador. Ampullaria, with Ceratodes, a peculiar planorbiform sub-genus, and Hemisinus, form the bulk of the fresh-water operculates.
The Galapagos.—Thirty-four species of land Mollusca, all peculiar, are known from these islands; 25 of these are forms of Bulimulus. There are no Helicidae, one each of Hyalinia, Leptinaria, and Helicina, and two Pupa. The Bulimulus are mostly of the group Nesiotis, and in their brown colour bear some outward resemblance to the dark Achatinella of the Sandwich Is., living as they do mostly under scoriae on the ground, and not on trees. In type, however, they appear to be derived from Chili and Peru, rather than from the parts of S. America immediately contiguous. Another section (Pleuropyrgus 2 sp.) closely resembles a marine Chemnitzia. The islands are all volcanic, and are probably not the result of subsidence; thus the existing species are not to be regarded as the relics of a more widespread fauna, but as a new set of inhabitants.
(4) The Brazilian Sub-region.—This immense district is very little known, except in the south, and it is consequently impossible to give any satisfactory account of its Mollusca. It is possible that eventually it will be found that it falls into provinces which correspond more or less to (a) the Amazon basin; (b) the mountainous district in the east, drained by the Tocantins and the San Francisco; (c) the Parana basin in the south central district; and (d) the Argentine or Pampas district in the extreme south. But at present the data are insufficient to establish any such subdivisions, whose existence, if proved, would have an important bearing on the problem of the coalescence of S. America into its present form.[379]
The Agnatha are represented by Streptaxis alone (17 sp.). Helix is rare, but includes the peculiar Polygyratia (Fig. 150 A, p. 246), while Labyrinthus (2 sp.), Solaropsis (5 sp.), and Systrophia[358] are common with the Colombian Sub-region, and Oxychona (4 sp.) with the Central American. Bulimus has in all 36 species, the sub-genera Pachyotus (Fig. 233) and Strophochilus being peculiar. Bulimulus, though not so abundant as in Peru and Ecuador, has about 60 species, of which Navicula (Fig. 235) is the most remarkable group. Megaspira is peculiar. Orthalicus has only 4 species, while Tomigerus (4 sp.) and Anostoma (3 sp.) are common with Venezuela. Land operculates are scarce, and appear to include only Neocyclotus, Cyclophorus, and Helicina.
In Argentina, which may probably rank as a separate province, the tropical forms greatly decrease, Streptaxis being reduced to 2 species, and Bulimus and Bulimulus together to 40, while Orthalicus, the great Helices, and the land operculates disappear altogether. Odontostomus (Fig. 236), a genus of the Pupidae, is abundant in the northern part of the province. Two or three species of Chilina occur.
Fig. 235.—Bulimulus (Navicula) navicula Wagn., Brazil.

Fig. 236.—Odontostomus pantagruelinus Moric., S. Brazil. × ½.
(5) The Chilian Sub-region.—The greater part of Chili, from its arid and rainless climate, is unfavourable to the existence of land Mollusca. Bulimus (Borus) still has 3 or 4 species, and Bulimulus (Plectostylus 11, Scutalus 9, Peronaeus 7) is fairly abundant, but the profusion of the tropics is wanting. There are no carnivorous genera, and only two land operculates. A remarkable form of Helix (Macrocyclis, Fig. 237) is quite peculiar, but the majority of the species belong to two rather obscure groups, Stepsanoda and Amphidoxa. Chilina, a singularly solid form of Limnaea (of which 8 sp., with a sub-genus Pseudochilina, occur in Chili), is peculiar to Chili, S. Brazil, and Patagonia. From the two islands of Juan Fernandez and Masafuera, are known several Helix, of Chilian affinity, several curious Succinea, a Homalonyx, Leptinaria, and Nothus, and three species of Tornatellina, with the almost universal Limax gagates.
The question of the existence at some remote period of a Neantarctic continent, which formed a communication between the three great southern peninsulas of the world, is one on[359] which the Mollusca may offer evidence. Von Ihering holds that an essential difference can be observed between certain of the Unionidae which inhabit S. America, Africa, and Australia with New Zealand, and those which inhabit Europe, Asia, and N. America, but the point can hardly be regarded as definitely established at present. Something perhaps may be made of the distribution of Bulimus and Bulimulus. It seems difficult to explain the occurrence of sub-fossil Bulimus on St. Helena except on some such lines as have been recently adduced to account for the presence of struthious birds in the Mascarenes, and possibly the form Livinhacea may be a trace of the same element in S. Africa. Again, the Liparus of S. and W. Australia, with the Caryodes of Tasmania, and the Leucotaenia and Clavator of Madagascar (which all may be related to Bulimus), together with the Placostylus of New Caledonia and the adjacent islands, reaching even to New Zealand, and perhaps even the Amphidromus of Malaysia (which are more akin to Bulimulus), may be thought to exhibit, in some remote degree, traces of a common ancestry.
The land operculates give no help, and, of the carnivorous genera, Rhytida is a marked link between Africa and Australia, while Streptaxis is equally so between S. America and Africa. As regards fresh-water Gasteropoda, Ampullaria is common to S. America and Africa, while Isidora is common to Africa, Australia, and New Zealand, but is altogether absent from S. America. Gundlachia occurs in Florida, Trinidad, and Tasmania, but has not been detected in Africa. It must be concluded, therefore, that the present state of the evidence which the Mollusca can afford, while exhibiting certain curious points of relationship between the three regions in question, is insufficient to warrant any decided conclusion.
Fig. 237.—Macrocyclis laxata Fér., Chili.
[360]
Marine Mollusca may be divided roughly into Pelagic and non-Pelagic genera. To the former division belong all Pteropoda and Heteropoda, and a large number of Cephalopoda, together with a very few specialised forms of Gasteropoda (Ianthina, Litiopa, Phyllirrhoe, etc.). Pelagic Mollusca appear, as a rule, to live at varying depths below the surface during the day, and to rise to the top only at night. The majority inhabit warm or tropical seas, though some are exceedingly abundant in the Arctic regions; Clione and Limacina have been noticed as far north as 72°.[380]
The vertical range of Pelagic Mollusca has received attention from Dr. Murray of the Challenger, Professor Agassiz of the Blake and Albatross, and others. Agassiz appears to have established the fact that the surface fauna of the sea is limited to a comparatively narrow belt of depth, and that there is no intermediate belt of animal life between creatures which live on or near the bottom and the surface fauna. Pelagic forms sink to avoid disturbances of various kinds, to depths not much exceeding 150 to 200 fathoms, except in closed seas like the Gulf of California and the Mediterranean, where the bathymetrical range appears to be much greater.[381]
Non-Pelagic Mollusca are, from one point of view, conveniently classified according to the different zones of depth at[361] which they occur. Thus we are enabled to distinguish Mollusca of (a) the littoral, (b) the laminarian, (c) the nullipore, or coralline, and (d) the abyssal zones. It must be borne in mind, however, that these zones cannot be exactly defined, and that while the littoral zone may be understood to imply the area between tide-marks, and the abyssal zone a depth of 500 fathoms and upwards, the limits between the laminarian and the coralline, and between the coralline and abyssal zones can only be fixed approximately.
The difficulty of assigning special genera or species to special ‘zones of depth’ is increased by two important facts in the phenomena of distribution. In the first place, it is found that species which occur in shallow water in northern seas often extend to very deep water in much lower latitudes. This interesting fact, which shows the importance of temperature in determining distribution, was first established by the dredgings of the Lightning and Porcupine off the western coasts of Europe. In the second place, a certain number of species seem equally at home in shallow and in abyssal waters, in cases where a great difference of latitude does not occur to equalise the temperature. Thus the Challenger found Venus mesodesma living on the beach (New Zealand) and at 1000 fath. (Tristan da Cunha); Lima multicostata in ‘shallow water’ (Tonga and Port Jackson) and at 1075 fath. (Bermuda); Scalaria acus from 49 to 1254 fath. (N. Atlantic); and S. hellenica from 40 to 1260 fath. (Canaries). The Lightning and Porcupine found, or record as found,[382] Anomia ephippium at 0 to 1450 fath., Pecten groenlandicus at 5 to 1785 fath., Lima subauriculata at 10 to 1785 fath., Modiolaria discors at 0 to 1785 fath., Crenella decussata at 0 to 1750 fath., Dacrydium vitreum at 30 to 2750 fath., Arca glacialis at 25 to 1620 fath., Astarte compressa at 3 to 2000 fath., and Scrobicularia longicallus at 20 to 2435 fath. Puncturella noachina has been found at 20 to 1095 fath., Natica groenlandica at 2 to 1290 fath., Rissoa tenuisculpta at 25 to 1095 fath. In many of these cases we are assured that no appreciable difference can be detected between specimens from the two extremes of depth.
In spite, however, of these remarkable vagaries on the part of certain species, we are enabled roughly to distinguish a large[362] number of genera as ‘shallow-water’ and ‘deep-water’ respectively, while a still larger number occupy an intermediate position. Among shallow-water genera may be named Patella, Littorina, Nassa, Purpura, Strombus, Haliotis, Mytilus, Cardium, Solen; while among deep-water genera are Pleurotoma, Scissurella, Seguenzia, Dentalium, Cadulus, Limopsis, Nucula, Leda, Lima, and Axinus.
Theories on the geographical distribution of marine Mollusca have been revolutionised by the discoveries of recent exploring expeditions. The principal have been those of Torell (Swedish) (1859–61) on the coasts of Greenland and Spitzbergen; of the Lightning and Porcupine (British) in 1868–70, in the N.E. Atlantic, off the Scotch, Irish, French, and Portuguese coasts, and in the Mediterranean; of the Challenger (British), under Sir C. Wyville Thomson, in 1873–76, in which all the great ocean basins were dredged or sounded; of the Blake (American), under Alexander Agassiz, in 1877–80, in the West Atlantic, Gulf of Mexico, and Caribbean Seas; of the Travailleur (French) in 1880–83, off the west coasts of France, Portugal, and Morocco, Madeira, the Canaries, and the Golfe du Lion; of the Talisman (French) in 1882, off the west coast of Africa from Tangier to Senegal, the Atlantic Islands, and the Sargasso Sea; of the Albatross (American) in 1891, off the west coast of tropical America; of several other vessels belonging to the U.S. Fish Commission and Coast Survey, off east American shores; and of the Prince of Monaco in the Hirondelle and Princesse Alice at the present time, in the N. Atlantic and Mediterranean.
The general result of these explorations has been to show that the marine fauna of very deep water is much the same all the world over, and that identical species occur at points as far removed as possible from one another. The ocean floor, in fact, with its uniform similarity of temperature, food, station, and general conditions of life, contains no effectual barrier to the almost indefinite spread of species.[383] To give a few instances. The Challenger dredged Silenia Sarsii in 1950 fath., 1100[363] miles south-west of Australia, and also in 2650 fath. off the mouth of the Rio de la Plata; Semele profundorum in 1125 fath. near the Canaries, and in 2900 fath. mid N. Pacific; Verticordia deshayesiana in 155 fath. near Cape York, and in 350 fath. off Pernambuco; Arca pteroessa in 2050 fath. mid N. Pacific, in 1000–1675 fath. west of the Azores, and in 390 fath. off the West Indies; Arca corpulenta in 1400 fath. off N.E. Australia, in 2425 fath. mid-Pacific, and in 1375 fath. near Juan Fernandez; Lima goliath in 775 fath. off S. Japan, and in 245 fath. off S. Patagonia; Pleurotoma engonia in 700 fath. north-east of New Zealand, and in 345 fath. off Inoshima. A surprising range was occasionally found even in shallow-water species; thus Petricola lapicida was discovered by the same expedition in the West Indies and N. Australia, Cardita calyculata off Teneriffe and in Bass Strait, Arca imbricata off Cape York and in the West Indies, Modiolaria cuneata at Port Jackson and Cape of Good Hope, Lima squamosa at Teneriffe and the Philippines. In these latter cases it is not improbable that the species lives in deep water as well, from which it has not yet been dredged.
It follows from these considerations that any attempt to classify marine Mollusca under Regions and Provinces can only apply to Mollusca which occur at moderate depths. The most important factor in the environment, as determining distribution, is the temperature of the water, which is probably to be regarded as affecting not so much the adult Mollusca as their ova; for the adult might possibly support life under conditions in which the ova would perish. It appears that a sudden change of temperature is the most effective barrier to distribution,[384] and may bring the range of a species to an almost instantaneous stop, while a very gradual change will allow it to extend its range very widely.
[364]
It has been usual to classify marine Mollusca from moderate depths under the following regions and sub-regions:—
| Regions | Sub-regions | |
| A. Atlantic and Circumpolar |
 |
1. Arctic. |
| 2. Boreal. | ||
| 3. Celtic. | ||
| 4. Lusitanian. | ||
| 5. West African. | ||
| 6. South African. | ||
| B. Indo-Pacific |  |
1. Indo-Pacific. |
| 2. Japanese. | ||
| C. Australian |  |
1. Australian. |
| 2. Neozealanian. | ||
| D. American |  |
1. Aleutian. |
| 2. Californian. | ||
| 3. Panamic. | ||
| 4. Peruvian. | ||
| 5. Magellanic. | ||
| 6. Argentinian. | ||
| 7. Caribbean. | ||
| 8. Transatlantic. |
includes the whole to the eastern shores of the Atlantic, from the extreme north of the Cape of Good Hope, together with the circumpolar seas, which may be regarded as roughly bounded by the Aleutian Islands and the coast of Newfoundland.
(1) The Arctic Sub-region includes the circumpolar seas, and is bounded in the N. Pacific by a line drawn between Cape Avinoff in Alaska, and Cape Lopatka in Kamchatka, so as to exclude the Aleutian Islands. On the western shores of the Atlantic the cold Labrador current brings it as far south as the coast of Newfoundland, but on the eastern shores the influence of the Gulf Stream has the contrary effect, so that the North Cape may be taken as its southern limit.
The principal genera (many species of which are common to the whole sub-region) are Volutomitra, Buccinum, Buccinopsis, Neptunea, Trophon, Bela, Admete, Velutina, Trichotropis, Lacuna, Margarita, Philine, Pecten, Leda, Yoldia, Astarte, and Mya. The shells are generally unicoloured, and of a dead white or rather sombre tint.
(2) The Boreal Sub-region may be subdivided into two provinces, the European and the American. The former includes the entire coast-line of Norway, the Färoe Islands, and Iceland (except perhaps the northern coast), and possibly the Shetland Islands; the latter the American coasts from the Gulf of St. Lawrence to Cape Cod (lat. 42°). Thus the Boreal American province does not extend nearly so far south as the Boreal[365] European, the reason being that on the American coasts the cold Labrador current, which hugs the land, bars back the advance of southern genera, but allows Boreal genera to spread southwards, while on the European side the warmer conditions produced by the Gulf Stream keep the Boreal species back, and allow more southern forms to spread northwards.
Many of the Boreal species occur on both sides of the Atlantic, and thus support the theory of a more continuous fringe of continental land once existing along the north of the Atlantic. Among the prominent genera, besides several of those mentioned under the Arctic Sub-region, are Purpura, Chenopus, Littorina, Gibbula, Natica, Patella, Tectura, Chiton, Doris, Aeolis, Tellina, Thracia.
(3) The Celtic Sub-region includes the British Islands (excepting perhaps the Shetland Islands), the coasts of the North Sea and the Baltic, with N. France to Cape Ushant. The absence of any cold or warm current exerting direct influence upon the coast-line of this sub-region causes a very gradual change in the conditions of life as we move either southward or northward. The fauna of the British seas contains a decided mixture of northern and southern forms. The following are among the common Boreal species which attain their southward range on our coasts: Tectura testudinalis Müll. (to Dublin Bay and Scarborough), Trichotropis borealis Brod. (to the Dogger Bank), Margarita helicina Fabr. (to Yorkshire and Dublin Bay), M. groenlandica Chem. (western Scotland), Natica montacuti Forb. (to Cornwall), Trophon truncatus Str. (to Tenby), Chiton marmoreus Fabr. (to Dublin Bay and Scarborough). Buccinum undatum and Littorina littorea become very scarce on our extreme south-western coasts. Among Lusitanian species which reach our coasts are Gibbula magus L. (to Orkney and Shetland Islands), Phasianella pullus L. (to Caithness), Galerus chinensis L. (to Milford Haven), Galeomma Turtoni Turt. (to Weymouth), Cardium aculeatum L. (to Isle of Man), Solen vagina L. (to north Ireland).
It appears from the Mollusca of our Crag formations that at the time of their deposition the temperature of our seas must have been considerably warmer than it is now. Thus in the Crag we find many species and even genera (e.g. Mitra, Fossarus, Triton, Vermetus, Ringicula, Chama) which now occur no farther[366] north than the southern coasts of the Channel, the west of France, and the Mediterranean.
The Baltic, a sea specially liable to violent changes of temperature, with a large admixture of fresh water at its eastern end, appears to possess only about 65 species in all. More than 50 genera occurring on the western coasts of Denmark do not enter the Sound. In the eastern portion of the Baltic marine and fresh-water species live together (p. 12).
(4) The Lusitanian Sub-region extends from Cape Ushant in the north to Cape Juby (lat. 28°) in the south, and includes the whole of the Mediterranean, as well as the Azores, Canaries, and Madeira groups.
The English Channel acts as an effectual barrier to the northward extension of many species; as many as 81 species which occur in western France do not reach British coasts (P. Fischer). At the same time, the western coasts of France are rather intermediate between the two sub-regions than distinctly Lusitanian, for between 50 and 60 Mediterranean genera do not occur on those coasts.
The Mediterranean itself is exceedingly rich in species, about 1200 in all (including deep-water species) being known. A certain number of these belong to tropical genera which here find their northern limit, e.g. Fasciolaria, Cancellaria, Sigaretus, Siliquaria, Chama, Spondylus. Here too occur Carinaria, Lobiger, Oxynoe, Pedicularia, Cypraea, Marginella, Mitra, Dolium, Cassis, Cassidaria, Pisania, Euthria, Vermetus, Argonauta, and many others. A few Celtic and even Boreal species, which occur on the western coasts of Morocco, do not enter the Mediterranean. Among these are Purpura lapillus, Helcion pellucidum, and Tellina balthica. Halia, a rare West African genus akin to Pleurotoma, is found in Cadiz Bay, and the West African Cymbium occurs on the Spanish coasts as far as Malaga.
The Black Sea, whose northern and western coasts are exceedingly cold, is comparatively poor in species. The Sea of Azof is chiefly characterised by forms of Cardium.
(5) The West African Sub-region extends from Cape Juby to a point probably not very far south of lat. 30° S., the cold current which sweeps up from the Pole probably limiting the southward extension of tropical species on this side of Africa, while the warm Mozambique current on the eastern side permits the[367] spread of many Indo-Pacific species almost as far south as the Cape. Owing to its extreme unhealthiness, and the absence of harbours, the sub-region is very little known.
The principal genera are Cymbium, Pleurotoma, Marginella, Terebra, Mitra, Agaronia, Murex, Cancellaria, Purpura, Pseudoliva, Natica, Tellina, Lucina, Tugonia, Schizodesma, and Arca. Studer has enumerated as many as 55 species common to West Africa and the opposite American shores. The north and south equatorial currents, which circulate in this part of the Atlantic, probably transport the larvae from one coast to the other. Purpura coronata Lam., a characteristic West African species, is represented by a well-marked variety in Demerara.
The Mollusca of St. Helena (178 known species) most resemble those of the West Indies, 50 per cent being common, while 30 per cent are common to the Mediterranean. From Ascension Island only 33 species are known, which in their general relations resemble those of St. Helena.[385]
(6) The South African Sub-region extends along the coast from about lat. 30° on the west, to about East London on the east. Mr. G. B. Sowerby enumerates 740 species from ‘South Africa,’ but includes in this list Natal species, which more properly belong to the Indo-Pacific fauna. Of these 740, 323 are peculiar, while 67 also occur in European seas, some being familiar on our own shores. It is remarkable to find in a sub-region separated from ourselves by the whole width of the tropics, such well-known forms as Mangilia costata Don., M. septangularis Mont., Cylichna cylindracea Penn., Pholas dactylus L., Solen marginatus Pult., Cultellus pellucidus Penn., Ceratisolen legumen L., Lutraria oblonga Chem., Tellina fabula Gmel., T. tenuis Da C., Modiolaria discors L., and many others.
The leading genera are Euthria, Triton, Cominella, Bullia, Nassa, Cypraeovula, Oxystele, Fissurella, Fissurellidaea, Patella, and Chiton.
The Mollusca of Kerguelen Island and the Marion and Crozets groups show relationship partly with South America, partly with the Cape, and partly with South Australia and New Zealand, thus showing some trace of a circumpolar antarctic fauna corresponding to, but not nearly so well marked as that of the circumpolar arctic sub-region. Among the remarkable forms[368] discovered off Kerguelen are Neobuccinum and a sub-genus of Struthiolaria (Perissodonta).
includes the whole of the coast-line of the Indian and western Pacific oceans, from about East London in South Africa to the north of Niphon (lat. 42°) in Japan, with the Red Sea and Persian Gulf, the whole of the Indo-Malay Archipelago, Polynesia to the Sandwich Islands in the north-east, and Easter Island in the south-east, and Australia to Swan River in the west, and to Sandy Cape and Lord Howe’s Island in the east. It is especially the region of coral reefs, which furnish so favourite a home of the Mollusca, and which are entirely absent from the Atlantic Region.
(1) The Indo-Pacific Sub-region proper (which includes the whole of this region except that part defined below as the Japanese Sub-region) is by far the richest in the world. The marine Mollusca of the Philippines alone (in some respects the nucleus of the whole region) have been estimated at between 5000 and 6000 species, and Jousseaume estimates Red Sea species at about 1000. Some prominent genera are very rich in species. Garrett enumerates from Polynesia 81 species of Conus, 60 of which occur on the Viti Is., 21 on the Sandwich Is., and only 14 on the Marquesas, where coral reefs are almost absent; 82 species of Cypraea, Viti Is. 44, Sandwich Is. 31, Marquesas only 13; 167 species of Mitra (besides 29 recorded by others), Viti Is. 120, Sandwich Is. 36, Marquesas 7. Of 50 existing species of Strombus, 39 occur in this region, and 10 out of 11 Eburna.
The following important genera are quite peculiar to the region: Nautilus, several forms of Purpuridae, e.g. Rapana, Magilus, Rapa, Melapium, and Ricinula; Tudicla, several forms of Strombidae, e.g. Rostellaria, Terebellum, Pteroceras, and Rimella; Cithara, Melo, Neritopsis, Stomatia, Malleus, Vulsella, Cucullaea, Tridacna, Hippopus, Libitina, Glaucomya, Anatina, Aspergillum, and many others.
The number of species common to the Red Sea and Mediterranean is exceedingly small, some authorities even denying the existence of a single common species. The present[369] author, from an examination of the shells dredged by MacAndrew at Suez, regarded 17 species as common, and Mr. E. A. Smith has confirmed this view with regard to 8 of the species in question.[386] The Mollusca occurring in Post-pliocene beds at Suez show that Mediterranean species lived there in comparatively recent geological times.
The opening of the Suez Canal appears to have already induced several species to start on their travels from the Mediterranean to the Red Sea and vice versâ. Two Red Sea species (Mactra olorina Phil., Mytilus variabilis Kr.) had in 1882 established themselves at Port Said, while two Mediterranean species (Pholas dactylus L., Solen vagina L.) had reached Ismailia.[387]
(2) The Japanese Sub-region consists of the Japanese Islands to Niphon, together with Corea and a stretch of adjacent mainland coast of unknown extent. The warm Kuro Siwo current, sweeping up between Luzon and Formosa, permits tropical species to extend much farther north than on the opposite shores of America, where a cold polar current keeps them back. A certain number of species, however, are common to the two shores of the Pacific, and a few circumpolar species occurring on our own coasts reach Japan, e.g.Trophon clathratus, Puncturella noachina, Mya arenaria, Modiola modiolus, Lasaea rubra, and Nucula tenuis.
Among the characteristic genera are Fusus, Siphonalia, Columbarium, Hemifusus, Rapana, Chlorostoma, Pleurotomaria, Haliotis, and Cyclina.
includes the Australian coast-line from about Swan R.[388] (lat. 32° S.) to Sandy Cape (lat. 25° S.), Tasmania, New Zealand, and the adjacent islands (except Lord Howe’s I.).
(1) The Australian Sub-region proper (which consists of the[370] whole of the region excepting New Zealand and the adjacent islands) is determined by the influence of the Antarctic Drift, which washes the whole of the southern coasts of Australia, and runs strongly northward between Australia and New Zealand. The E. Australian warm current from the north is checked at Sandy Cape by this cold current, and flows off to New Zealand, the western shores of which island are consequently much warmer than the eastern. On the western coast of Australia the Antarctic Drift has less force, and tropical genera accordingly range some 7 degrees farther south on the western than on the eastern coasts.
The characteristic genera are Voluta (of which half the known species occur on Australian coasts[389]), Cominella, Siphonalia, Struthiolaria, Risella, Phasianella, a number of genera belonging to the Trochidae, e.g. Liotia, Clanculus, Euchelus, Thalotia, Elenchus, Trochocochlea, Zizyphinus, Bankivia, Trigonia, Myodora, Myochama, Solenomya, Ephippodonta, Anapa, Mylitta, Mesodesma, and Chamostrea. Trigonia, originally discovered as a recent form in Sydney Harbour (p. 65), is not peculiar to that locality, occurring also off Cape York, West Australia, and Tasmania.
(2) The Neozealanian Sub-region includes New Zealand, with the outlying islands (Chatham, Auckland, and Campbell Is.).
As many as 455 species (Cephalopoda, 8; Gasteropoda, 311; Scaphopoda, 2; Pelecypoda, 134) have been enumerated by Professor F. W. Hutton as occurring within the sub-region, of which only 64 are found elsewhere, the proportion of peculiar species being thus nearly 86 per cent. New Zealand therefore is, in its marine, no less than its land Mollusca, greatly isolated.
The characteristic genera are Anthora, Cryptoconchus, and Vanganella, which appear to be quite peculiar, Trophon, Cominella, Euthria, most of the Trochidae also characteristic of S. Australia, Haliotis, Patella, Taria, Mesodesma, Mylitta, Zenatia, Standella, and Myodora.
includes the entire coasts of North and South America with the adjacent islands, south of Cape Avinoff on the western, and south[371] of Cape Cod on the eastern coast, the portions north of these points belonging to the Arctic Sub-region.
(1) The Aleutian Sub-region consists of the islands of Yesso and Saghalien, with the adjacent shores of the Sea of Okhotsk to Cape Lopatka, the Aleutian Is., and the west American coast from about Cape Avinoff (lat. 60° N.) to St. Jean de Fuca Straits.
A certain number of species, probably of arctic origin, are common with British and also with East American shores, the former being the more numerous. Species as familiar to us as Lacuna divaricata Fabr., Trichotropis borealis Brod., Pholas crispata L., Mya truncata L., M. arenaria L., Mytilus edulis L., and Modiolaria nigra Gray, occur. The more characteristic genera are Chrysodomus, Volutharpa, Buccinum, Tectura, Scurria, Chiton, Cryptochiton (Cr. Stelleri Midd. is by far the largest known of the Chitonidae, 6 inches long), Tellina and Pecten.
(2) The Californian Sub-region extends from St. Jean de Fuca Straits (lat. 48° N.) to Cape St. Lucas, the Gulf of California belonging to the Panamic sub-region. The northern polar current, which washes the shores of this sub-region throughout their whole extent, prolongs the southward range of the more northern genera, and keeps back those more markedly tropical, the latter, however, creeping northward in the warmer waters of the Gulf of California. Some authorities subdivide this immense stretch of coast-line, as characterised by sub-temperate, temperate, and sub-tropical genera, into the Oregonian, Californian, and Lower Californian provinces.
The characteristic genera are—in the north, Argobuccinum, Zizyphinus, Chlorostoma, Tectura, Scurria, Chiton (Katharina, Mopalia, Tonicia), Cryptochiton, Placunanomia, and Mytilimeria; in the centre, Purpura, Monoceros, Amphissa, Norrisia, Platyodon, Tapes, and Macoma; and, towards the south, Olivella, Chorus, Macron, Pseudoliva, Trivia, and Haliotis.
(3) The Panamic Sub-region extends from the head of the Gulf of California to Payta in Peru (lat. 5° S.). It is exceedingly rich in species, about 1500 having been described. The Mollusca are entirely distinct from those of the Indo-Pacific Region, which, although extending from Natal to the Sandwich Islands, are unable to pass the enormous extent of sea which separates the nearest Polynesian island from the American coast.
On the two sides of the isthmus of Panama there occur certain[372] pairs of species, which, while specifically distant, are evidently closely related to one another. Amongst these are, on the Panamic side, Purpura speciosa, Cypraea cervinetta, Cassis abbreviata, Natica Chemnitzii, and Strombus gracilior, corresponding to Purpura deltoidea, Cypraea exanthema, Cassis inflata, Natica maroccana, and Strombus pugilis, on the Caribbean. It is reasonable to conclude that these “analogous species” are descendants of a stock which was common to both seas when the isthmus was open (probably not later than Miocene times), and which have, since the closing of the isthmus, become modified, some species considerably more than others.
Among the characteristic genera (compare p. 3) are Conus, Pleurotoma, Terebra, Murex, Purpura, Oliva, Northia, Cantharus, Columbella, Anachis, Cypraea, Strombus, Cerithium, Coecum, Crepidula, Crucibulum, Vitrinella; Tellina, Semele, Tellidora; and Arca.
(4) The Peruvian Sub-region extends from Payta in Peru to about the latitude of Conception in S. Chili (37° S.), being checked from further extension southward by the cold Humboldt current, whose force is distinctly felt as far north as Callao. This cold current thus produces the same results as the similar current which impinges on S. Africa, but has even more effect in decisively separating the fauna on the two sides of the great peninsula, scarcely a single species being common to the western and eastern coasts of S. America. The characteristics of the coast-lines themselves contribute to this result. The Chilian coast is rocky, and descends abruptly to a great depth, while that of Patagonia and Argentina is sandy and very shallow to a great distance from land.
The characteristic genera are Cancellaria, Columbella, Monoceros, Concholepas, Trochita, Fissurella, Chiton; Ceronia, Malletia, and Cumingia. Some of the Californian genera, absent or poorly represented in the Panamic Sub-region, reappear in Chili, e.g. Scurria, Tectura, and Chlorostoma.
(5) The Magellanic Sub-region includes the coast-line and adjacent islands (with the Falklands) from Conception in S. Chili to about Port Melo in Eastern Patagonia (lat. 45° S.).
The principal genera (many of which find a habitat on the gigantic Macrocystis which grows on every rock at low water) are Euthria, Voluta (6 species, one, V. magellanica, the largest known)[373] Monoceros, Photinula, Patella, Chiton; Modiolarca, Malletia, and Mulinia. Several genera characteristic of the Boreal and Arctic sub-regions recur, e.g. Trophon, Admete, Margarita, Puncturella, Cyamium, and Astarte.
(6) The Argentinian[390] Sub-region extends from about Cape Melo in Patagonia to the neighbourhood of S. Caterina I. in South Brazil (lat. 28° S.). The sub-region stands in the same relation to the Magellanic, on the east coast, as the Peruvian sub-region on the west, but, owing to the influence of the warm Brazil current, which overpowers the colder water of the Falkland branch of the Cape Horn current, it reaches a point much farther south.
The Mollusca are not well known. The prevailing genera appear to be Oliva, Olivancillaria, Voluta, Bullia, Crepidula; Periploma, and Lyonsia.
(7) The Caribbean Sub-region extends from S. Caterina I. in the south to Florida in the north, and includes the shores of the Gulf of Mexico and the whole of the West Indies. The influence of the warm Brazil current (a branch of the South Equatorial) carries the range of the purely tropical species to a point much farther south than is reached by the tropical species on the west coast.
The sub-region is very rich in species, especially on the coral reefs of the Bahamas and N. Cuba, but the exceedingly small tide-fall makes shore collecting somewhat difficult beyond a certain point. The leading genera are Murex, Purpura, Melongena, Latirus, Marginella, Strombus, Triton, Cerithium, Littorina, Nerita, Scalaria; Tellina, Strigilla, Lucina, and Venus. Pleurotomaria, a genus long regarded as extinct, has been dredged alive off Tobago.
As compared with the tropical fauna of the Old World, that of the New World is poor in peculiar genera (compare p. 368). The relations of this sub-region to the West African and the Panamic have been already dealt with (pp. 367 and 372).
(8) The Transatlantic Sub-region extends from Florida to Cape Cod (see p. 364). In the north the limits of the sub-region are distinctly marked, in the south Caribbean species intermingle.[374] Gould and Binney, in their Invertebrata of Massachusetts, enumerate 275 species (Cephalopoda, 6; Gasteropoda, 159; Scaphopoda, 2; Pelecypoda, 108), of which 59 (Gasteropoda, 37; Pelecypoda, 22) are British.
Among the characteristic genera are Urosalpinx, Eupleura, Fulgur, Ptychatractus, Nassa, Crepidula; Solenomya, Mactra, Cypricia, Raëta, Astarte, and Yoldia. Our common Littorina littorea appears to have been introduced into Nova Scotian waters in about 1857, no previous trace of it occurring either in literature or shell-heaps. Since then it has spread rapidly into the Gulf of St. Lawrence, and also as far south as Newhaven, and is said to be driving out the indigenous L. palliata from New England shores.[391] The debt has been repaid by the introduction into British waters of the American clam (Venus mercenaria L.), which, according to the Manchester City News of 23rd March 1889, was first observed in the Humber in 1864, and has steadily increased up to the present time, when it bids fair to compete, in those waters, with the familiar Cardium edule.
Characteristics of Abyssal Mollusca.—Large shells appear to be rare in the great ocean depths, and are usually very fragile; even moderately-sized specimens are far from common. The only group in which species occur larger than the usual size is the Nudibranchs, which are represented by at least one form larger than an orange.
It would seem that abyssal molluscs are much less active and energetic than their brethren on the shores. This view is favoured by the looseness of their tissues, which seem ill adapted for prompt and vigorous action. The tenacious character of the mud on the ocean floor must make rapid motion very difficult. The shell itself is usually fragile and delicate, the upper layers of arragonite being thin as compared with shallow-water species, which makes the nacreous layer, when present, appear unusually conspicuous; in many cases the surface is characterised by a peculiar iridescence or sheen. The colour in the shell of deep-sea Mollusca is never very pronounced, and is often absent altogether. Light pink and salmon, pale yellow and brown, are not uncommon. If the colour is in pattern, it is usually in the form of necklaces of spots, which sometimes coalesce into bands. With regard to sculpture, stout knobs and powerfully buttressed varices, such as[375] occur in the tropical Murex and Purpura, are not found in deep-sea species. But the ornamentation is frequently elaborate, and the sculpture rich and varied. There is an especial tendency towards strings of bead-like knobs, revolving striae, and delicate transverse waves, the sculpture being in many cases of a character which tends to strengthen the structure of the shell, like the ridges in corrugated iron.
A remarkable feature in some deep-sea Mollusca is their singular resemblance, in shape, and particularly in the possession of a strong green periostracum, to some of our common fresh-water species. According to Dr. Dall, the cause of this phenomenon is the same in both cases. The fresh-water Mollusca secrete a strong periostracum, in order to protect the shell against the corrosive influence of the carbonic acid gas with which the water is surcharged. The shells of deep-sea Mollusca, living, as they do, in water probably undisturbed by currents of any kind, have to protect themselves against the same eroding influence, and do so in the same way.[392]
Mollusca which live exclusively on algae and other forms of plant life are almost entirely wanting in the great depths, where vegetation is probably unknown. The struggle for existence must be much less keen than in the thickly populated shallows, where vicissitudes of every kind occur. The absence of rapid motion of water must obliterate many of those mechanical effects which tend to produce modifying influences upon the animals affected. In the absence of circumstances tending to cause variation, in the unbroken monotony of their surroundings, species must, one would think, preserve a marked uniformity over an exceedingly wide area of range.
Vegetable food being wanting, those genera which in shallower waters never taste flesh, are compelled to become carnivorous. Characteristic of the great depths are very remarkable forms of Trochidae, in whose stomachs have been found the remains of Corallines and Foraminifera. According to Dr. Dall, the results of this diet show themselves in the greatly increased space occupied by the intestine, in the diminution, as regards size, of the masticatory organs, the teeth and jaws, and also in the prolongation of the anal end of the intestine into a free tube, which carries away the excreta in such a way that they do not foul the[376] water taken into the gills. The amount of nutriment contained in the bodies of dead Foraminifera is so small that a comparatively large quantity must be swallowed to keep the vital energies active, and therefore the amount evacuated must be proportionately larger also. The abyssal Trochidae, then, and many other genera, sustain themselves by feeding on the ‘rain’ of dead animal matter which falls upon the ocean floor, not so much hunting their prey as opening their mouths and eating whatever happens to fall into them. Genera which are normally carnivorous would appear to do the same. The Pleurotomidae, for instance, are a family markedly characteristic of very deep water. Representatives of the genus which occur in shallower water are known to secure their prey while in the living state. But, according to Dr. Dall, a singularly small proportion of deep-sea Mollusca, as compared with those from the littoral region, show signs of having been drilled or attacked by other Mollusca. This could hardly be the case if the Pleurotomidae retained their predatory habits, since they are more numerous in the great depths than any six other families taken together. It has already been mentioned (p. 186) that a large proportion of deep-sea Mollusca are perfectly blind.
Amongst other remarkable forms from the great depths may be mentioned Pleurotomaria, with its singular anal slit (Fig. 269, p. 407) extending in some cases half way round the last whorl. Three or four species of this genus, so characteristic of almost all fossiliferous strata down to the Cambrian, have been obtained in very limited numbers off the West Indies and Japan. Dentaliidae, especially the sub-genus Cadulus, find a congenial home in the slimy ocean mud. One of the greatest molluscan treasures procured by the Challenger was Guivillea alabastrina Wats., a magnificent Volute as white as alabaster, 6½ inches long, which was dredged from 1600 fath. in the South Atlantic, between Marion Island and the Crozets. Another very curious form, belonging to the same family, is Provocator pulcher Wats., a shell about half the size of Guivillea, of stouter proportions, and with an angulated and patulous mouth. This shell was dredged by the Challenger in comparatively shallow water (105–150 fath.) off Kerguelen Island. Among the Trochidae are the fine new genera Basilissa, Bembix, and Gaza. The exploring voyages of the American surveying steamer Blake, in the Gulf of Mexico and the Caribbean[377] Sea, have given us the remarkable new forms Benthobia (possibly akin to Admete), Mesorhytis (a sub-genus of Fasciolaria hitherto only known from the Cretaceous of North America), and Benthodolium (possibly = Oocorys), a genus akin to Cassis.
In his report on the Pelecypoda obtained by the Challenger, Mr. E. A. Smith remarks that as a rule “very deep-water ‘benthal’ species certainly have a tendency to be without colour and of thin structure, facts no doubt resulting from the absence of light, the difficulty of secreting lime, the scarcity of food, and other unfavourable conditions of existence.” At the same time, he notices that most of the species obtained belong to genera which, even when occurring in shallow water, are thin and colourless, e.g. Neaera, Lima, Cryptodon, Abra, Verticordia, etc. Deep-water species of such genera as have a decided periostracum (Malletia, Limopsis, Leda, Nucula, Arca) retain it with little if any modification. The deep-water Pelecypoda of the Atlantic and Pacific Oceans present no special features of interest. The species are few in number, and the genera are not remarkable either for novelty or peculiarity of form.
The greatest depth at which Pelecypoda have been obtained is 2900 fath. mid North Pacific (Callocardia pacifica Sm., Abra profundorum Sm.); the greatest depth at which Gasteropoda have been obtained is 2650 fath. South Atlantic (Stylifer brychius Wats.), both by the Challenger. The deepest Challenger Nudibranch came from 2425 fath., and the deepest Chiton from 2300 fath. The greatest depth ever dredged is 4575 fath. off the east coast of Japan.
[378]
The Cephalopoda present a complete contrast to the majority of the Mollusca in habits and in many points of organisation. In their power of rapid movement and their means of progression, their extreme ferocity and carnivorous habits, their loss, in so many cases, of a shell, and in its constitution when present, in the general symmetry of their parts, in their reproductive and nervous system, they stand in a position of extreme isolation with nothing to connect them with the rest of the phylum.
Professor A. E. Verrill has collected many interesting details with regard to gigantic Cephalopoda occurring on the north-eastern coasts of America. From these it appears that the tentacular arms of some species of Architeuthis measure as much as 32, 33, 35, and 42 feet in length, while the total length, including the body, sometimes exceeds 50 feet. Even off the Irish coast a specimen was once captured whose tentacular arms were 30 feet long, the mandibles 4 inches across, and the eyes about 15 inches in diameter.[393] The strength of these giant Cephalopods, aided as they are by formidable rows of suckers and other means of securing a grip, is almost incredible. Cases are not uncommon, in which persons diving or bathing have been attacked, and have with difficulty made their escape.
Great damage is frequently inflicted by Cephalopoda upon shoals of fish on British coasts. Off Lybster (Caithness) Loligo and Ommastrephes devour the herring, large numbers of which are cut up and bitten on the back of the neck by these creatures. On the American coasts the mackerel fisheries are sometimes entirely spoiled by the immense schools of squid which infest the[379] Bay of St. Lawrence.[394] When excited in the pursuit of fish Cephalopoda leap high out of the sea. Dr. W. H. Rush[395] relates that when about 300 miles off the coast of Brazil, a swarm of hundreds of decapods flew from the water and landed on the deck of the ship, which was 12 feet above the surface level, and they had to go over the hammock nettings to reach it.
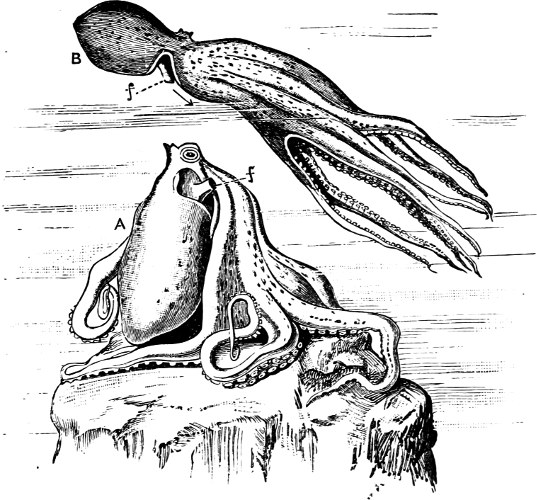
Fig. 238.—Octopus vulgaris Lam., Naples: A, At rest; B, in motion; f, funnel, the arrow showing the direction of the propelling current of water. (After Merculiano.)
The common Octopus vulgaris Lam., of British and south European coasts, inhabits some rocky hole, the approaches to which, like the den of a fabled giant, are strewn with the bones of his victims. Homer himself knew how hard it is to drag the polypus out of his hole, and how the stones cling fast to his suckers. The colour-changes, which flit across the skin of the Octopus, appear, to some extent, expressive of the different emotions of the animal. They are also undoubtedly protective, enabling it to assimilate itself in colour to its environment. Mr. J. Hornell[396] has noticed an Octopus, while crawling over the rock-work in his tank, suddenly change the colour of the whole right or left side of its body, and of the four arms on the same side, to a snowy whiteness. They have also been seen to change colour, as if involuntarily, according to the material on which they crawl.[380] The nerve-centres which control the chromatophores or pigment cells, causing them to expand or contract, are found to connect with the optic ganglia; hence the changes of colour may be regarded as a reflex result of the creature’s visual perception of its surroundings.
Cephalopoda with two symmetrical branchiae, funnel completely tubular, mouth surrounded by 8 or 10 arms furnished with suckers or hooks, ink-sac and fins usually present, eyes with a lens; shell internal or absent.
The Dibranchiata are not known from Palaeozoic strata, and first appear (Belemnites, Belemnoteuthis) in the Trias. Whether they are to be regarded as derived from some form of Tetrabranchiata, e.g. Orthoceras, or as possessing an independent origin from some common stock, cannot at present be decided. They attain their highest development at the present time. The earliest representatives of the Order (the Phragmophora) possessed a shell chambered like that of the Tetrabranchiata. These chambered Dibranchiates rapidly reached their maximum in the upper Lias and as rapidly declined, until at the close of the Cretaceous epoch they were comparatively scarce, only a few genera (Beloptera, Spirulirostra) surviving into Tertiary times.
The ordinary Dibranchiate Cephalopod may be regarded as consisting of two parts—(a) the head, in which are situated the organs of sense, and to which are appended the prehensile organs and the principal organs of locomotion; (b) a trunk or visceral sac, enclosed in a muscular mantle and containing the respiratory, generative, and digestive organs. The visceral sac is often strengthened, and the viscera protected, by an internal non-spiral shell. The ‘arms’ which surround the mouth are modifications of the molluscan foot (p. 200), and are either eight or ten in number. In the former case (Octopoda) the arms, which are termed ‘sessile,’ are all of similar formation, in the latter (Decapoda), besides the eight sessile arms there are two much longer ‘tentacular’ arms, which widen at their tips into ‘clubs’ covered with suckers.
Remarks have already been made on the generative organs of Cephalopoda (p. 136 f.), the branchiae (p. 170), the nervous[381] system (p. 206), the eye (p. 182), the radula (p. 236), and the ink-sac (p. 241).
One of the most characteristic features of the Dibranchiata are the acetabula, or suckers, with which the arms are furnished. They are usually disposed on the sessile arms in rows (of which there are four in most Sepia, two in Octopus, and one in Eledone), and become more numerous and smaller at the tip of the arm. They are massed together in large numbers of unequal size on the ‘clubs’ in the Decapoda, particularly in Loligo. In most Octopoda their base is flush with the surface of the arm, but in Decapoda the acetabula are pedunculate, or raised on short stalks. In Octopoda again, the acetabula are fleshy throughout, but in the Decapoda they are strengthened by a corneous rim with a smooth or denticulate edge (Ommastrephes, Architeuthis). Many of the acetabula on the tentacular and sometimes on the sessile arms of the Onychoteuthidae enclose a powerful hook, which is retractile like the claws of a cat.
In mechanical structure the acetabula consist of a disc with a slightly swollen margin, from which a series of muscular folds converge towards the centre of the disc, where a round aperture leads to a gradually widening cavity. Within this cavity is a sort of button, the caruncle, which can be elevated or depressed like the piston of a syringe; thus when the sucker is applied the piston is withdrawn and a vacuum created (Owen).
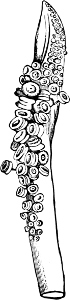
Fig. 239.—‘Club’ of Loligo vulgaris L., showing the crowded pedunculate acetabula, × ½.
Fig. 240.—One of the suckers of Architeuthis dux Stp., showing the denticulate margin and corneous ring; p, peduncle.
In many Octopoda the arms are connected by a web (the umbrella), which sometimes extends up the greater part of the arms (Cirrhoteuthis, some Eledone), at others occurs only at the base. The use of the umbrella is perhaps to assist in locomotion, by alternate contraction and expansion.
A cartilaginous skeleton is well developed, especially in the Decapoda.
In Sepia a cephalic cartilage forms a complete ring round the
oesophagus, the eyes being situated in lateral prolongations[382] of
the same. In front of the cephalic cartilage occurs a piece like an
inverted 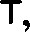 , which supports the base of the anterior arms. The
Decapoda have also a ‘nuchal’ cartilage, connecting the head with the
anterior dorsal portion of the mantle, while cartilaginous knobs on the
ventral mantle button into corresponding sockets on the funnel.
, which supports the base of the anterior arms. The
Decapoda have also a ‘nuchal’ cartilage, connecting the head with the
anterior dorsal portion of the mantle, while cartilaginous knobs on the
ventral mantle button into corresponding sockets on the funnel.
Sub-order I.—Octopoda.—Body round or bag-like, generally without fins, arms eight, suckers fleshy, usually sessile, oviducts paired, no nidamental glands, shell absent.
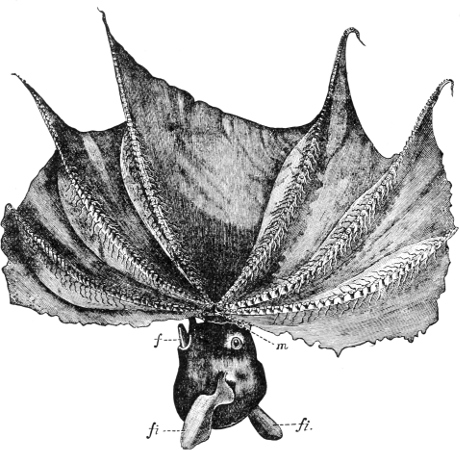
Fig. 241.—Cirrhoteuthis magna Hoyle, S. Atlantic. Two of the left arms and their web have been removed: f, funnel; fi, fi, fins; m, mouth. (After Hoyle, × 1/12.)
Fam. 1. Cirrhoteuthidae.—Body with two prominent fins; arms in great part united by a web; one row of small suckers, with cirrhi on each side.—Atlantic and Pacific Oceans, deep water (Fig. 241).
Fam. 2. Amphitretidae.—Body gelatinous, mantle fused with[383] the funnel in the median line, forming two openings into the branchial cavity; arms with one row of suckers; umbrella extending more than two-thirds up the arms.—South Pacific (Fig. 242).
The two pocket-like openings into the branchial cavity are unique among Cephalopoda (Hoyle).
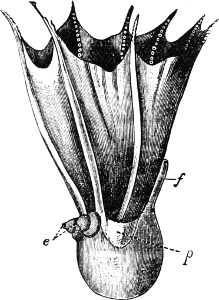
Fig. 242.—Amphitretus pelagicus Hoyle, off Kermadec Is.: e, eyes; f, funnel; p, right mantle-pocket. (After Hoyle.)
Fam. 3. Argonautidae.—Female furnished with a symmetrical, unilocular shell, spiral in one plane, secreted by thin terminal expansions (the vela) of the two dorsal arms, no attachment muscle; suckers in two rows, pedunculate; male very small, without veligerous arms or shell.—All warm seas (Fig. 243). Pliocene——.
The shell consists of three layers, the two external being prismatic, the middle fibrous. Its secretion by the arms and not by the mantle edge is unique, and shows that it is not homologous with the ordinary molluscan shell.
Fig. 243.—Argonauta argo L., the position assumed by a specimen kept in captivity, the arrow showing the direction of movement: f, funnel; m, mouth, with jaws projecting; sh, shell, with arms as seen through it; wa, webbed arm clasping shell. (After Lacaze-Duthiers.)
The great controversy on the Argonauta, which once raged with so much fierceness, is now matter of ancient history. It seems scarcely credible that between fifty and sixty years ago, two of the leading zoologists of the day, Mr. Gray and M. de[384] Blainville maintained that the animal which inhabits the Argonaut shell is a parasite, without any means of depositing or forming a shell of its own, but which possesses itself of the Argonaut shell, either by expelling or succeeding the original inhabitant, a supposed nucleo-branchiate (Heteropod) mollusc akin to Carinaria. The final blow to this strange hypothesis—which was urged by the most ingenious series of arguments—was given by Professor Owen, who in 1839 brought before the Zoological Society of London the admirable observations of Madame Jeannette Power, who made a continuous study of a number of specimens of Argonauta in her vivarium at Messina. The result of these observations tended to show that the young Argonauta when first excluded from the egg is naked, but that in ten or twelve days the shell begins to form, that the principal agents in the deposition of shell are the two velated or web-like arms; and that portions of the shell, if broken away, are repaired by a deposition of calcareous matter.[397]
Fam. 4. Philonexidae.—Mantle supported by two ridges placed on the funnel; large ‘aquiferous’ pores (supposed to introduce water into the tissues) near the head or funnel; suckers in two rows, pedunculate.—Atlantic and Mediterranean.
Genera: Ocythoe, arms of unequal size, no intervening membrane, third arm on the right hectocotylised (see Fig. 51, p. 138), two aquiferous pores at the base of the siphon; male very small; Tremoctopus, two aquiferous pores between the eyes, two on the ventral side of the head.
Fam. 5. Alloposidae.—Mantle edge united to the head by three commissures; arms extensively webbed, acetabula sessile. Hectocotylised arm developed in a cavity in front of the right eye.—N. Atlantic.
Fam. 6. Octopodidae.—Head very large, arms elongated, similar, more or less webbed, acetabula usually in two rows, sessile; mantle supported by fleshy bands, no cephalic aquiferous pores.
In Octopus proper the web is usually confined to the lower part of the arms; Fischer separates off as Pteroctopus a form in which it reaches almost to their extremity. The third right arm (Fig. 52, p. 140) is hectocotylised, the modified extremity being, according to Hoyle, sometimes minute, sometimes spoon-shaped, with a tendency to transverse ridges, rarely slender and[385] very long. The relative length of the pairs of arms varies in different species. Two cartilaginous stylets, imbedded in the dorsal mantle, are said by Owen to represent the shell.
Other genera; Pinnoctopus, body furnished with broad lateral wings which meet at the posterior end; Cistopus, a large web prolonged along the sides of the arms, fitted with oval aquiferous pouches, with pores at their base, between each pair of arms; Eledone (Fig. 244), one row of acetabula; Tritaxeopus, Iapetella.
Sub-order II.—Decapoda.—Body oblong, mouth surrounded by four pairs of sessile and one pair of tentacular arms, the latter terminated by a ‘club’; acetabula pedunculate and furnished with a corneous margin; mantle margin locked to the base of the funnel by a cartilaginous apparatus; head and anterior part of body furnished with aquiferous pores; fins present; mandibles corneous; oviduct single, large nidamental glands in the female; shell internal.
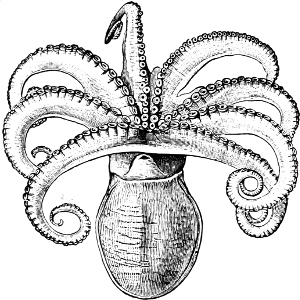
Fig. 244.—Eledone Aldrovandi Delle Chiaje, Naples, from ventral side, × ½.
The tentacular arms, which are the principal external feature of the Decapoda, are not derived from the same muscular ring as the sessile arms, but arise from the cephalic cartilage, and emerge between the third and fourth arm on each side. In Sepia they can be entirely retracted into a kind of pocket behind the eyes, while in Loligo they are simply folded over one another. In Chiroteuthis the arms are six times as long as the body, and the clubs have four rows of denticulate suckers.
The anterior ventral[398] portion of the mantle is furnished with a singular contrivance for locking it to the funnel, and so rendering the whole animal more capable of resisting the impact of any force. This contrivance generally consists of a series of ridges or buttons which fit into grooves or button-holes, the ridges being on the interior face of the mantle and the corresponding grooves[386] on the funnel, or vice versâ. The ‘resisting apparatus’ is most elaborate in the pelagic genera, and least so in the more sluggish littoral forms. A similar, but not so complex, arrangement occurs also in the Octopoda.
The different forms of the shell appear to indicate successive stages in a regular course of development. We have in Spirula (Fig. 247) a chambered shell of the Tetrabranchiate type, but of considerably diminished size, which has ceased to contain the animal in its last chamber, and has become almost entirely enveloped in reflected folds of the mantle. These folds gradually concresce to form a definite shell-sac, by the walls of which are secreted additional laminae of calcareous shell-substance. These laminae invest the original shell, which gradually (Spirulirostra, Belosepia) loses the spiral form and becomes straight, eventually disappearing, while the calcareous laminae alone remain (Sepia). These in their turn disappear, leaving only the plate or ‘pen’ upon which they were deposited (Loligo), which itself also, with the shell-sac, finally disappears, surviving only in the early stages of Octopus (Lankester).
The Decapoda are divided, according to the character of the shell, into Phragmophora, Sepiophora, and Chondrophora.[399]

Fig. 245.—‘Club’ of Onychoteuthis sp., showing the hooks and clusters of fixing cushions and acetabula below them, × ½.
A. Phragmophora.—Arms furnished with hooks or acetabula; shell consisting of a phragmocone or chambered sac enclosed in a thin wall (the conotheca), septa pierced by a siphuncle near the ventral margin (in Spirula alone this chambered sac forms the whole of the shell). The apex of the cone lies towards the posterior end of the body, and is usually enveloped in a calcareous guard or rostrum. Beyond the anterior end of the rostrum the conotheca is extended forward dorsally by a pro-ostracum or anterior shell, which may be shelly or horny, and corresponds to the gladius of the Chondrophora. The rostrum consists of calcareous fibres arranged perpendicularly to the planes of the laminae of growth, and radiating from an axis, the so-called[387] apical line, which extends from the extremity of the phragmocone to that of the rostrum. Distribution, see p. 380.
Fam. 1. Spirulidae.—Arms with acetabula, shell a loose spiral, without rostrum or pro-ostracum, partially external, enclosed in two lobes of the mantle (Figs. 247 and 248).
The single species of the single genus (S. Peronii Lam. = laevis Gray) has not yet been thoroughly investigated, although the shell occurs in thousands on many tropical beaches, and is sometimes drifted on our own shores. The animal appears to have the power of adhesion to the rocks by means of a terminal sucker or pore. The protoconch is present, and contains a prosiphon, which does not connect with the siphuncle. The septal necks are continuous, not broken as in Nautilus. The siphuncle is on the ventral margin of the shell, the last whorl of which projects slightly on the dorsal and ventral sides, but is even there covered by a thin fold of the mantle. The retractor muscles of the funnel and of the head find their point d’appui on the shell, the last chamber of which contains the posterior part of the liver, with which the membranous siphuncle is connected.
Fam. 2. Belemnitidae.—Arms hooked as in Onychoteuthis, fins large; phragmocone straight, initial chamber globular, larger than the second, rostrum often very long, investing the phragmocone, pro-ostracum sword- or leaf-shaped, rounded in front, seldom preserved, ink-sac present.—Lower Lias to Cretaceous.
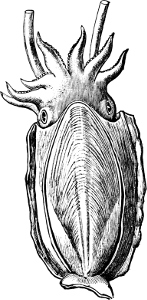
Fig. 246.—Sepia officinalis L., with mantle cut away to show position of internal shell, × ½. (The ends of the tentacular arms are cut off.)
The Belemnitidae are believed to have been gregarious, and to have lived in shallow water on a muddy bottom. Specimens are sometimes found in which even the ink-sac can be recognised in situ. The relative proportions of rostrum and phragmocone vary greatly in different groups, the rostrum being in some cases two feet long, in others only just enclosing the phragmocone.[388] As a rule the rostrum is the only portion which has been preserved.
Fam. 3. Belosepiidae.—Phragmocone short, slightly curved, chambers small, placed at the posterior end of a sepion, rostrum solid, obtuse.—Eocene (Paris, Bracklesham, etc.).
Fam. 4. Belopteridae.—Sepion not known; phragmocone curved, siphuncle on the ventral margin, rostrum well developed, pointed. Principal genus, Spirulirostra.—Miocene of Turin.
These two families, with their small, curved phragmocone and (in the case of the Belosepiidae) large sepion, are clearly intermediate between the Phragmophora and Sepiophora. Some authorities place them with the latter group.
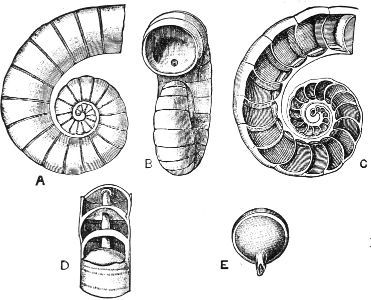
Fig. 247.—Shell of Spirula Peronii Lam. A, Cutside view; B, showing last chamber and position of siphuncle; C, in section, showing the septa and course of siphuncle; D, shell broken to show the convexity of the inner side of the septa; E, portion of a septal neck.
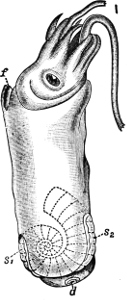
Fig. 248.—Spirula Peronii Lam.: d, terminal sucker; f, funnel; s1, s2, projecting portions of shell, the internal part of which is dotted in. (From Owen and A. Adams combined.)
B. Sepiophora.—Shell internal, consisting usually of (a) an anterior cancellated portion, (b) a posterior laminated portion, the laminae enclosing air. It terminates in a very rudimentary phragmocone and a rostrum, but there is no siphuncle.
Fam. Sepiidae.—Eyes with cornea complete, body oval, fins narrow, lateral, as long as the body, generally united behind; sessile arms short, tentacular arms long, acetabula generally in[389] four rows, fourth left arm in the male hectocotylised near the base (Fig. 249).—World-wide.
The sepion or ‘cuttle-bone’ runs the whole length and width of the body. In Sepia it is very thick in front, while the posterior ventral end is concave and terminated by a prominent spine, the rostrum or mucro which points downwards. The whole shell is surrounded by a thin chitinous margin, which forms a lateral expansion. Other genera are Sepiella, Hemisepius, and Trachyteuthis (fossil only).
C. Chondrophora.—Shell (gladius or pen) long, chitinous.
(a) Myopsidae:[400] cornea entire, species mostly sub-littoral.
Fam. 1. Sepiolidae.—Fins large, dorso-lateral; tentacular arms retractile; two first dorsal arms in the male hectocotylised; gladius narrow, half as long as the body.—World-wide.
Principal genera: Sepiola, dorsal mantle connected with the head by a broad cervical band, ventral mantle with the funnel by a ridge fitting into a groove; Rossia, dorsal mantle supported by a ridge, arms with never more than four rows of acetabula; Inioteuthis, Stoloteuthis, Nectoteuthis, and Promachoteuthis.
Fam. 2. Sepiadariidae.—Fins not as long as the body, mantle united to the head on the dorsal side, fourth left arm in the male hectocotylised; no gladius. Principal genera, Sepiadarium, Sepioloidea.—Chiefly Pacific Ocean.
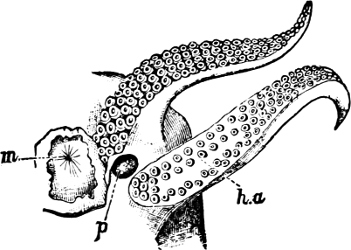
Fig. 249.—Hectocotylised arm (h.a.) of Sepia officinalis L., shown in contrast to one of the ordinary sessile arms; m, mouth; p, pocket into which the tentacular arm is retracted.
Fam. 3. Idiosepiidae.—Fins very small, terminal; fourth pair of arms in the male hectocotylised, bare of suckers.
The only genus, Idiosepion, with a single species (I. pygmaeum Stp.) is from the Indian Ocean, and is the smallest known Cephalopod, measuring only about 15 mm. in length.
Fam. 4. Loliginidae.—Body rather long, fins varying in size, tentacular arms partially retractile, gladius as long as the back, pointed in front, shaft keeled on the ventral side.—World-wide.
Loligo proper has a pointed body with triangular posterior fins united behind; sessile arms with two rows of acetabula,[390] tentacular arms with four; fourth left arm hectocotylised at the tip; funnel attached to the head. Other genera are Loliguncula, Sepioteuthis, and Loliolus. Belemnosepia, Beloteuthis, Leptoteuthis, and Phylloteuthis are fossil genera only, differing in the shape of the gladius.
(b) Oigopsidae: cornea more or less open; species pelagic.
Fam. 5. Ommastrephidae.—Body cylindrical, fins generally terminal, united together, regularly rhomboidal, sessile arms with varying number of rows of acetabula, mantle connexions elaborate; gladius horny, narrow lanceolate, with a hollow cone at the posterior end.—World-wide.
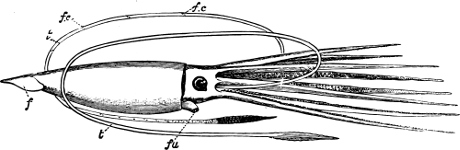
Fig. 250.—Architeuthis princeps, Verr., E. America: f, Right fin; fu, funnel; f.c, fixing cushions and acetabula on the tentacular arms (t, t). (After Verrill, × 1/60.)
Ommastrephes proper has a natatory web on the sessile arms; the wrist of each club has a series of acetabula with corresponding cushions on the other wrist. In Thysanoteuthis (often made a separate family) the sessile arms have two rows of cirrhi, with lateral expansions of the skin; fins as long as the body. In Architeuthis, to which belong the largest Cephalopoda known, the fins together are shaped like a broad arrow-head; acetabula of sessile arms strongly denticulate; tentacular arms very long, with equidistant pairs of acetabula and fixing cushions throughout their entire length, and a group of the same at the base of the club. The acetabula and cushions correspond on the opposing tentacles, and enable them to pull together. Other genera are Dosidicus, Todarodes, Illex, Bathyteuthis and Mastigoteuthis.
Fam. 6. Onychoteuthidae.—Body cylindrical, fins terminal or lateral, mantle-locking apparatus elaborate, tentacular arms very long, sessile or tentacular arms furnished with retractile hooks, gladius lanceolate, with a terminal cone.—World-wide.
The prehensile apparatus of Cephalopoda reaches its maximum of power and singularity in this family. In Onychia, Onychoteuthis[391] and Ancistroteuthis, the sessile arms have acetabula only, in Gonatus and Abralia they have hooks as well, while in Verania, Ancistrochirus and Enoploteuthis, the sessile arms have hooks only. The number of rows of hooks or acetabula varies with the different genera.
Fam. 7. Chiroteuthidae.—Head nearly as large as the body; fins terminal, tentacular arms very long, sessile arms slightly webbed, acetabula denticulated; mantle-supports consisting of cartilaginous ridges on the mantle, which fit into corresponding depressions on the funnel, gladius expanded at each end.—Atlantic Ocean.
The six dorsal arms in Histioteuthis are united by a broad web, while in Histiopsis the web only reaches half way up the arm. In Chiroteuthis the tentacular arms have scattered sessile suckers throughout their whole length, and four rows of very long pedunculate suckers on the clubs.
Fam. 8. Cranchiidae.—Head small, body rounded, barrel-shaped, fins terminal, eyes often very large, sessile arms short, tentacular arms long, thread-like.—World-wide.
Cranchia proper has the tentacular clubs finned, with eight rows of suckers, body sometimes covered with warty tubercles. Loligopsis has a very attenuated body, with fins terminally united; some species are spotted with colour, or have rows of tubercles on the ventral side. Taonius (Fig. 251) is doubtfully distinct from Loligopsis.
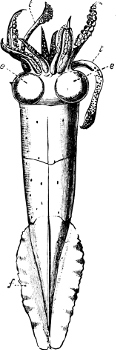
Fig. 251.—Taonius hyperboreus Stp., N. Atlantic: e, e, eyes; f, f, fins; t, t, tentacular arms. (After Hoyle, × ¼.)
Cephalopoda with four branchiae and four kidneys; animal inhabiting the last chamber of an external multilocular shell;[392] funnel consisting of two separate lobes; tentacles numerous, without suckers or hooks; no ink-sac.
The shell consists of two layers, the outer being porcellanous, and the inner, as well as the walls of the chambers or septa, nacreous. The septa vary greatly in shape. In most of the Nautiloidea they are regularly curved, as in Nautilus, or straight, as in Orthoceras, but in the Ammonoidea they are often exceedingly complex. The edge of the septum, where it unites with the shell-wall, is called the suture, and the sutural line, which is not seen until the porcellanous layer is removed, varies in shape with the septum.
Fig. 252.—Nautilus pompilius L., in section, showing the septa (s, s), the septal necks (s.n, s.n), the siphuncle dotted in (si), and the large body chamber (ch).
The septa are traversed by a membranous tube known as the siphuncle, which in Nautilus is said by Owen to connect ultimately with the pericardium. The septal necks, or short tubular prolongations of the septa where they are perforated by the siphuncle, are in the great majority of the Nautiloidea directed backwards (Fig. 252), i.e., they project from the front wall of each chamber, while in nearly all Ammonoidea they are directed forwards. When the siphuncle is narrow, as in the Ammonoidea, it is simple, but when wide, as in many of the Nautiloidea,[393] its walls are often thickened by the deposition of masses of calcareous matter, or by rings and radiating lamellae of the same material. In position, the siphuncle is sometimes central, sometimes sub-central, sometimes (Ammonoidea) marginal. In some cases its position is believed to change during the growth of the individual. The precise object served by the siphuncle is at present unknown. Some hold that it preserves the vitality of the unoccupied chambers, by connecting them with the soft parts of the animal; others have regarded it as a means for lightening the shell by the passage of some gas into the chambers.
Fig. 253.—Ammonites (Cadoceras) sublaevis Sowb., Kellaway’s Rock, showing the marginal position of the siphuncle (si).
The initial chamber in Nautiloidea consists of an obtuse incurved cone, marked on the outer surface of its posterior wall by a small scar known as the cicatrix, which may be slit-like, round, oval, or cruciform in shape. It has been held that the cicatrix originally communicated with the protoconch or larval shell, which probably dropped off as development proceeded. In the Ammonoidea, on the other hand, there is no cicatrix, and the initial chamber probably represents the protoconch, as seen in the nucleus of many Gasteropoda.
Sub-order 1. Nautiloidea.—Shell straight, bent, or coiled, aperture simple or contracted; siphuncle often narrowed by internal deposits, position variable; septal necks short, usually directed backwards; septa concave towards the aperture; initial chamber conical, with a cicatrix on the posterior wall.
The Nautiloidea, of which Nautilus is the sole living representative, date back to the Cambrian epoch, and attain their maximum in the Silurian and Devonian. At the close of the Palaeozoic era, every family, with the sole exceptions of the Orthoceratidae and Nautilidae, appears to have become extinct. The former disappear with the Trias, and after the lapse of the whole Secondary era, Aturia, a form closely related to Nautilus, makes its appearance.
(a) Retrosiphonata: septal necks directed backwards.
Fam. 1. Orthoceratidae.[401]—Shell straight or slightly curved, aperture simple, body-chamber large; siphuncle cylindrical,[394] position variable. Single genus, Orthoceras (Fig. 254). Cambrian to Trias.
Fam. 2. Endoceratidae.—Shell straight, siphuncle wide, marginal, septal necks produced into tubes fitting into one another. Principal genera: Endoceras (specimens of which occur six feet long), and Piloceras—Ordovician.
Fam. 3. Actinoceratidae.—Shell straight or slightly curved, siphuncle wide, contracted at the septa by obstruction-rings. Principal genera: Actinoceras, Discosorus, Huronia, Sactoceras.—Ordovician to Carboniferous.
Fam. 4. Gomphoceratidae.—Shell globular, straight or considerably curved, aperture narrowed, T-shaped, body-chamber large, siphuncle variable in position. The aperture is in some cases so narrow that probably only the arms could be protruded. Principal genus, Gomphoceras (Fig. 255).—Silurian.
Fam. 5. Ascoceratidae.—Shell sac-like or flask-shaped, apex truncated, unknown, body-chamber occupying nearly the whole of the shell on the ventral side, contracting at the aperture, last few septa coalescing on the dorsal side and encroaching upon the body-chamber. The young form has a symmetrical shell like Orthoceras, attached to the sac-like shell above described; as growth proceeds the former portion is thrown off. Principal genera: Ascoceras, Glossoceras.—Ordovician and Silurian.
Fig. 254.—A, Section of Orthoceras, showing the septa (s, s), and siphuncle (si, si); B, portion of the exterior of Orthoceras annulatum Sowb., × ½. (Woodwardian Museum, Cambridge.)
Fam. 6. Poterioceratidae.—Shell fusiform, contracted at both ends, aperture simple, siphuncle variable in position, inflated between the septa. The form generally resembles Gomphoceras, except for the simple aperture and fusiform shape.—Ordovician to Carboniferous.
Fam. 7. Cyrtoceratidae.—Shell conical or sub-cylindrical, slightly curved, body-chamber large, siphuncle variable in position. Single genus, Cyrtoceras.—Cambrian to Carboniferous.
Fam. 8. Lituitidae.—Shell coiled in a flat, sometimes loose[395] spiral, last whorl straight, containing the body-chamber, often greatly prolonged. Principal genera: Lituites, Ophidioceras.—Ordovician and Silurian.
Fam. 9. Trochoceratidae.—Shell helicoid, with seldom more than two whorls, dextral or sinistral, last whorl sometimes partly uncoiled. Principal genera: Trochoceras, Adelphoceras.—Ordovician to Devonian.
Fam. 10. Nautilidae.—Shell with few whorls, more or less overlapping, septa simple, siphuncle central or sub-central, aperture not contracted.
The ‘tentacles’ are about 90 in number, and consist of four groups each of 12 or 13 labial tentacles surrounding the mouth, two groups each of 17 larger (brachial) tentacles on each side of the head, two thicker tentacles which combine to form the ‘hood,’ and two small tentacles on each side of the eye. When the animal swims, the tentacles are extended radially from the head, somewhat like those of a sea-anemone. The direction of the many pairs of tentacles at constant but different angles from the head, is the most striking feature in the living Nautilus, and accounts for its being described, when seen on the surface, as ‘a shell with something like a cauliflower sticking out of it.’[402] The funnel is not a complete tube, but is formed by the overlapping of the margins of two thin fleshy lobes (which are probably morphologically epipodia), so that when the two lobes are parted, a broad canal appears, leading to the branchial cavity. The head is conical, and the mouth and its appendages can be retracted into a sort of sheath, over which fits the ‘hood.’
Fig. 255.—A, Gomphoceras ellipticum M’Coy, Silurian: B, aperture (ap) of same; s, s, septa; si, position of siphuncle. (After Blake.)
Other genera are Trocholites, Gyroceras, Hercoceras, Discites, Aturia.—Ordovician to present time.
Fam. 11. Bactritidae.—Shell straight, conical, siphuncle small, marginal, septal necks long, funnel-shaped, sutures undulating, with a sinus corresponding to the siphuncle. This family, from the form of its sutures, appears to constitute a passage to the Ammonoidea. Single genus, Bactrites.—Silurian and Devonian.
(b) Prosiphonata.—Septal necks directed forwards.
The two genera are Bathmoceras (Ordovician), shell straight,[396] conical always truncated, siphon marginal; and Nothoceras (Silurian), shell nautiloid with simple sutures.
Sub-order 2. Ammonoidea.—Shell multiform, straight, curved, flat spiral, or turreted, sutural line more or less complex, siphuncle simple.
Some authorities hold that the members of this great sub-order, now totally extinct, belong to the Dibranchiata, on the ground that the protoconch resembles that of Spirula rather than that of the Nautiloidea. Others again regard the Ammonoidea as a third, and distinct Order of Cephalopoda. Their distribution extends from the Silurian to (possibly) the early Tertiary. No trace has ever been found of an ink-sac, mandible, or hooks on the arms; the shell was undoubtedly external.
Fig. 256.—Diagram of the sutures of Ammonites: A, an elaborate suture (Phylloceras); B, a simple suture (Ceratites); s.s, siphonal, s.v, ventral, s.l, first lateral, s.l´, second lateral saddles; s.a, s.a, auxiliary saddles; l.v, ventral, l, first lateral, l´, second lateral lobe; l.a, l.a, auxiliary lobes. The arrow points towards the aperture. (From Woodward.) Compare Fig. 258.
The sutural line, which indicates the septa, and is generally concealed beneath the outer layer of shell, consists of a number of lobes or depressions, the concave part of which is directed towards the aperture. Between these lobes lie corresponding elevations, or saddles, the convex part of which is directed towards the aperture. There are six principal lobes (Fig. 256): the siphonal or ventral, which is traversed by the siphuncle, the dorsal, and a superior and inferior lateral on each side; smaller auxiliary lobes may succeed these latter. The adjacent saddles have received corresponding names. As a rule the sutural line is very complex, but in some cases (Goniatites, Lobites) it is simple (Fig. 258, A). The first saddle of a large number of genera serves as a means of classification, according as it is broad or narrow. Some authorities reverse the terms ventral and dorsal, as applied above. It is probable, however, that the position of[397] the animal of Ammonites in its shell resembled that of Nautilus. The siphuncle is dorsal (internal) in Clymenia only, ventral (external) in all other genera.
The aptychus of Ammonoidea is a corneous or calcareous valve-like body, generally formed of two symmetrical parts (Fig. 257). It has been regarded by some as the covering of the nidamental gland, and hence as occurring only in the female, by others, with more probability, as an operculum, covering or imbedded in a hood formed, as in Nautilus, of modified arms. Sometimes the Aptychus is in a single piece (Anaptychus), sometimes the two pieces are united on the median line (Synaptychus).
Fig. 257.—Aptychus of Ammonite (Trigonellites latus). Kimmeridge Clay, Ely. × ½.
The Ammonoidea are thus classified by Dr. P. Fischer:—
| (a) Retrosiphonata | Goniatitidae. | ||
| (b) Prosiphonata | No Aptychus or Anaptychus corneous, single |
First saddle, wide |
Arcestidae, Tropitidae, Ceratitidae, Clydonitidae. |
| First saddle, narrow |
Pinacoceratidae, Amaltheidae, Ammonitidae, Lytoceratidae. |
||
| Aptychus calcareous, valves double or united |
Harpoceratidae, Stephanoceratidae. | ||
(a) Retrosiphonata. Fam. 1. Goniatitidae.—Shell nautiloid, whorls sometimes disjoined, siphuncle ventral or dorsal, sutures simple. Principal genera: Clymenia, Goniatites (Fig. 258, A).—Devonian to Carboniferous.
(b) Prosiphonata. Fam. 2. Arcestidae.—Shell globular, smooth or striated and rayed, body-chamber very long, aperture often with a projecting hood, umbilicus closed by a callosity, lobes numerous, foliaceous, aptychus present. Principal genera: Arcestes, Lobites.—Principally Trias.
Fam. 3. Tropitidae.—Differs from Arcestidae mainly in the more highly ornamented surface, which is decorated with ribs which become granular at the periphery. Principal genus, Tropites.—Trias and Lias.
Fam. 4. Ceratitidae.—Shell ribbed and tuberculated, body-chamber short, lobes denticulated, saddles simple. Principal genera: Ceratites (Fig. 258, B), Trachyceras.—Principally Trias.
Fam. 5. Clydonitidae.—Shell variable in form, body-chamber[398] short, sutural line undulated, simple. Principal genera: Clydonites, Choristoceras, Rhabdoceras, Cochloceras.—Trias.
Fam. 6. Pinacoceratidae.—Shell discoidal, usually smooth, body-chamber short, sutural line very complex, lobes numerous. Principal genera: Pinacoceras, Sageceras.—Carboniferous to Trias.
Fam. 7. Amaltheidae.—Shell broad, keeled, last whorl concealing most of the spire, sutures with auxiliary lobes, incised.—Principal genera: Amaltheus, Schloenbacia, Sphenodiscus.—Trias, Cretaceous.
Fig. 258.—Various forms of Ammonoidea: A, Goniatites crenistria J. Phil., Carb. Limestone; B, Ceratites nodosus de Hann., Muschelkalk; C, Ammonites (Parkinsonia) Parkinsoni Sowb., Inf. Oolite; D, Phylloceras helerophyllum Sowb., Upper Lias; s, s, sutural lines.
Fam. 8. Ammonitidae.—Body-chamber long, whorls narrow, uncovered, more or less ribbed, aperture simple, sutural line normal, aptychus single, corneous. Principal genera: Ammonites, Aegoceras.—Principally Lias.
Fam. 9. Lytoceratidae.—Shell discoidal, body-chamber short, aperture simple, no aptychus. Principal genera: Lytoceras, Phylloceras (Fig. 258, D).—Trias to Cretaceous.
Fam. 10. Harpoceratidae.—Shell discoidal, compressed, margin keeled, surface with straight or arched ribs, aperture[399] with lateral projections, suture with accessory lobes, aptychus in two pieces. Principal genera: Harpoceras, Oppelia, Lissoceras.—Jurassic to Cretaceous.
Fig. 259.—A, Turrilites catenulatus d’Orb, Gault; B, Macroscaphites Iranii d’Orb, Upper Neocomian. (From Zittel.)
Fam. 11. Stephanoceratidae.—Shell discoidal, helicoid or straight, whorls sometimes disunited, surface often with bifurcating ribs, which are tubercled, aperture often with lateral projections, sutural line incised, aptychus in two pieces, sometimes united.
In the discoidal group, Stephanoceras is strongly ribbed, tubercled at the point of bifurcation, Cosmoceras has long lateral projections of the aperture when young, Perisphinctes has a large body-chamber and numerous smooth ribs. Other genera are Acanthoceras, Peltoceras, Aspidoceras, and Hoplites. Among the loosely whorled genera, Scaphites (Fig. 260, A) has the last whorl produced and bent back again in horse-shoe form, while the early whorls are concealed; Hamites, Hamulina, and Ptychoceras have a shell shaped like a single or double hook, the sides of which may or may not be united; Crioceras (Fig. 260, B) in form of whorls resembles a Spirula, Ancyloceras a Scaphites with the first whorls disunited. Macroscaphites (Fig. 259, B) is similar, but with the first whorls united and not concealed. Turrilites (Fig. 259, A) is turreted and sinistral, while Baculites is quite straight, with a long body-chamber.
Fig. 260.—A, Scaphites aequalis Sowb., Cretaceous; B, Crioceras bifurcatum Quenst., Cretaceous. (From Zittel.)
[400]
Bilaterally symmetrical Mollusca, anus at the terminal end of the body, dorsal tegument more or less furnished with spicules.
Sub-order 1. Polyplacophora (Chitons).—Foot co-extensive with ventral surface of the body, dorsum with eight transverse plates, articulated (except in Chitonellus), a row of ctenidia on each side between the mantle and the foot. Silurian ——.
The Chitons are found in all parts of the world, ranging in size from a length of about half an inch to six inches or more in the giant Cryptochiton. Although in the main sub-littoral, they occur at very great depths; the Challenger dredged Leptochiton benthus Hadd. at 2300 fathoms. Chiton Polii exceptionally occurs at Malta—teste MacAndrew—above sea margin, but within reach of the ripple. As a rule, the Chitons live in concealment, on the under surface of stones or in deep and narrow fissures in the rocks. When the stone to which they are attached is turned over, they crawl slowly to the side which is not exposed, as if disliking the light. An undescribed species, however, which I took at Panama, crawled quite as fast as an ordinary snail. Chiton fulvus Wood, apparently is accustomed to crawl with some rapidity. MacAndrew took it in abundance on his anchor chain in Vigo Bay every time his yacht was got under weigh. He also found it crawling in sand on the shore, to which habit is no doubt due its extreme cleanness and freedom from the foreign growths which are so characteristic of many of the species. When detached a Chiton contracts the muscles of the whole body, and rolls up into a ball like a wood-louse.
[401]
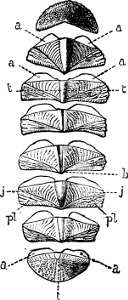
Fig. 261.—Valves of a Chiton separated to show the various parts (anterior valve uppermost): a, a, articulamentum; b, beak; j, jugum; pl, pl, pleura; t, t, tegmentum.
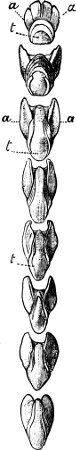
Fig. 262.—Valves of Chitonellus separated out (anterior valve uppermost): a, a, articulamentum; t, t, tegmentum. × 2.
The Polyplacophora are characterised, externally, by their usually articulated shell of eight plates or valves, which is surrounded and partly kept in position by a muscular girdle. These plates overlap like tiles on a roof in such a way that the posterior edge of the first, cephalic, or anterior valve projects over the anterior edge of the succeeding valve, which in its turn overlaps the next, and so on throughout. Seven-valved monstrosities very rarely occur.
A certain portion of each valve is covered either by the girdle or by the valve next anterior to it. This portion, which is whitish in colour and non-porous in structure, forms part of an inner layer which underlies the rest of the substance of the valve, and is called the articulamentum. The external portion of the valves, or tegmentum, is generally more or less sculptured, and is largely composed of chitin, impregnated with salts of lime, thus answering more to a cuticle than to a shell proper. It is very porous, being pierced by a quantity of minute holes of two sizes, known as megalopores and micropores, which are connected together by minute canals containing what is probably fibrous or nerve tissue, the mouths of the pores being occupied by sense organs connected with these nerves. The tegmentum of the six intermediate valves is generally divided into three triangular areas by two more or less prominent ribs,[402] which diverge from the neighbourhood of the median beak or umbo. The space enclosed between these ribs is known as the median area or jugum, the other two spaces as the lateral areas or pleura. The ribs terminate with the edge of the tegmentum, and are not found on the articulamentum. In certain genera these areas are either non-existent, or are not distinctly marked. The sculpture of the lateral areas (which is, as a rule, much stronger than that of the median area) will generally be found to resemble that of the anterior valve, which has no proper median area. In the posterior valve the median area is very small, while the sculpture of the rest of the valve corresponds to that of the lateral areas generally (see Fig. 261).
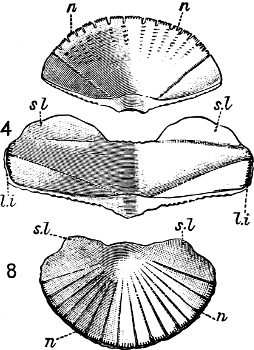
Fig. 263.—First, fourth, and eighth valves of a Chiton, showing l.i, laminae of insertion; n, n, notches; s.l, s.l, sutural laminae. × 2.
The articulamentum of the intermediate valves is divided into two equal parts in the middle of the anterior edge, opposite to the beak, by a sinus. Each of the portions thus formed is again divided by a notch or suture into two unequal parts, the anterior of which is known as the sutural lamina, and is more or less concealed by the valve in front of it, while the lateral part, or lamina of insertion, is entirely concealed by the girdle. The articulamenta of the anterior and posterior valves are either simple or pierced by a series of notches (Fig. 263).
The girdle of the Chitonidae varies considerably in character. Sometimes its upper surface is simply corneous or cartilaginoid, with no other sculpture than fine striae, at others it is densely beset with spines or bristles, or tufted at intervals with bunches of deciduous hairs; again it is marbled like shagreen or mossy down, or covered with serpent-like scales. The width of the girdle varies greatly, being sometimes very narrow, sometimes entirely covering all the valves (Cryptochiton). As a rule, its outer edge is continuous, but in Schizochiton it is sharply notched over the anus.
[403]
A description has already been given of the dorsal eyes in Chiton (p. 187), the nervous system (p. 202), the branchiae (p. 154), the radula (p. 228), and the generative system (p. 126).
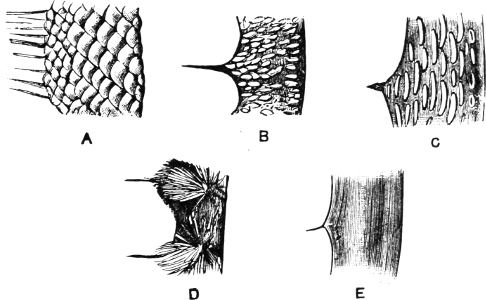
Fig. 264.—Girdles of various Chitonidae. A, Radsia sulcata Wood, × 2; B, Maugeria granulata Gmel., × 3; C, Enoplochiton niger Barnes, × 3; D, Acanthochiton fascicularis L., × 4; E, Tonicia fastigiata Sowb., × 4.
The recent Chitons are thus classified by Dr. W. H. Dall:—
Section I. Chitones Regulares.—Anterior and posterior valves of similar character.
A. Leptoidea.—Insertion plates obsolete, or, if present, unslit; Leptochiton, Hanleyia, Hemiarthrum, Microplax.
B. Ischnoidea.—Insertion plates sharp, smooth, fissured; with eaves; Trachydermon, Callochiton, Tonicella, Schizoplax, Leptoplax, Chaetopleura, Spongiochiton, Ischnochiton, Callistochiton.
C. Lophyroidea.—Insertion plates broad, pectinated, projecting backward; Chiton, Tonicia, Eudoxochiton, Craspedochiton.
D. Acanthoidea.—Insertion plates thrown forward; Sclerochiton, Acanthopleura, Dinoplax, Middendorffia, Nuttallina, Arthuria, Phacellopleura.
Section II. Chitones Irregulares.—Posterior valve abnormal, or with a sinus behind.
E. Schizoidea.—Posterior valve fissured; Lorica, Schizochiton.
F. Placiphoroidea.—Posterior valve unslit, internally ridged, umbo nearly terminal; Enoplochiton, Ornithochiton, Plaxiphora.
G. Mopaloidea.—Posterior valve with posterior sinus and one slit on each side; Mopalia, Katherina, Acanthochiton, Notoplax.
[404]
H. Cryptoidea.—With double sutural laminae; Cryptoconchus, Amicula, Cryptochiton.
I. Chitonelloidea.—Posterior valve funnel shaped; laminae thrown forward; Chitonellus, Choneplax.
Fig. 265.—Chitonellus fasciatus Quoy; ant, anterior end.
Sub-order 2. Aplacophora.—Animal vermiform, foot absent, or a mere groove, cuticle more or less covered with spicules.
According to Marion, one of the principal authorities on the group, the Aplacophora are perhaps Amphineura whose development has been arrested at an early stage, their worm-like exterior being due to adaptation to surroundings. They have hitherto been found chiefly in the N. Atlantic and Mediterranean, generally at considerable depths, and often associated with certain polyps in a way which suggests a kind of commensalism.
Fam. 1. Neomeniidae.—Foot a narrow groove, intestinal tube without differentiated liver, kidneys with common exterior orifice, sexes united, ctenidia present or absent. Genera: Neomenia (Fig. 266), Paramenia, Proneomenia, Ismenia, Lepidomenia, Dondersia.
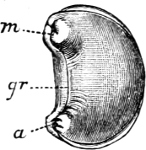
Fig. 266.—Neomenia carinata Tullb.: a, anus; gr, ventral groove; m, mouth.

Fig. 267.—Chaetoderma nitidulum Lov.: a, anus; m, mouth. × 3.
Fam. 2. Chaetodermatidae.—Body cylindrical, no ventral groove, liver a single sac, kidneys with separate orifices into the branchial cloaca, two bipectinate ctenidia. Single genus, Chaetoderma (Fig. 267).
Visceral loop twisted into a figure of 8 (streptoneurous), right[405] half supra-intestinal, left half infra-intestinal; heart usually in front of the branchia (ctenidium), which is generally single; head with a single pair of tentacles; animal dioecious, usually marine, more or less contained within a shell, operculum generally present. Cambrian to present time.
Sub-order 1. Diotocardia.—Heart with two auricles (except in the Docoglossa and Helicinidae), branchiae bipectinate, front end free; two kidneys, the genital gland opening into the right (except in Neritidae); nervous system not concentrated; no proboscis or siphon, penis usually absent.
(a) Docoglossa (p. 227).—Heart with a single auricle, ventricle not traversed by the rectum, visceral sac not spiral, shell widely conical, non-spiral, no operculum; radula very long, with few hooked teeth in each row.
Fam. 1. Acmaeidae.—Left ctenidium alone occurring, free on a long stalk. Cretaceous——. Principal genera: Pectinodonta, front part of head much produced, radula 0 (1. 0. 1.) 0; Acmaea (= Tectura), with sub-genera Collisella and Collisellina, no accessory branchial ring, shell closely resembling that of Patella, but generally with a distinct internal border; Scurria, accessory branchial ring on the mantle.
Fam. 2. Lepetidae.—No ctenidia or accessory branchiae, animal generally blind. Pliocene——. Principal genera: Lepeta; Propilidium, apex with internal septum; Lepetella.
Fam. 3. Patellidae.—No ctenidia, the osphradial patch at the base of each alone surviving, a circlet of secondary branchiae between the mantle and sides of the foot. Ordovician——. (i.) Patellinae.—Three lateral teeth on each side, two of them anterior. Principal genera: Patella, branchial circlet complete; chief sections Patella proper, Scutellastra, Ancistromesus (A. mexicana Brod., measures 8–14 in. long); Helcion, branchial circlet interrupted in front; Tryblidium (Ordovician).—(ii.) Nacellinae.—Two developed laterals on each side, one anterior. Genera: Nacella, branchial circlet complete; Helcioniscus, branchial circlet interrupted in front.
(b) Rhipidoglossa (p. 225).—Ventricle of the heart traversed by the rectum (except in Helicinidae), one or two ctenidia; jaw in two pieces, radula long, marginals multiplied, rows curved.
Of all the Gasteropoda, this section of the Diotocardia approach nearest to the Pelecypoda, particularly in the least[406] specialised forms. The auricle, the branchiae, and the kidneys are in many cases paired, and more or less symmetrical. The ventricle is generally traversed by the rectum, there is a long labial commissure between the cerebral ganglia, special copulative organs are usually absent, while the shell is often nacreous, like those of Pelecypoda of a primitive type.
Section I. Zygobranchiata.—Two ctenidia, shell with apical or marginal slit or holes, corresponding to an anal tube in the mantle (p. 265).
Fam. 1. Fissurellidae.—Two symmetrical ctenidia and kidneys, visceral mass conical, shell conical, elevated or depressed, with a single anterior or apical slit or impression; no operculum. Jurassic——. (i.) Fissurellinae. Shell wholly external, apex entirely removed by perforation, apical callus not truncated posteriorly; central tooth narrow. Genera: Fissurella (Figs. 171, p. 261; 178, p. 265), Fissuridea, Clypidella. (ii.) Fissurellidinae. Shell partly internal, otherwise as in (i.); central tooth broad, mantle more or less reflected over the shell, apical hole very wide. Genera: Fissurellidaea, Pupillaea, Lucapina, Megatebennus, Macroschisma, Lucapinella. (iii.) Emarginulinae. Shell usually wholly external, apex usually not removed by perforation, sometimes with internal septum, anal tube in a narrow slit or sinus. Genera: Glyphis, externals of Fissurella, but holecallus truncated behind; Puncturella (sub-genera Cranopsis and Fissurisepta), slit just anterior to the apex, a small internal septum; Zeidora, large internal septum as in Crepidula: Emarginula, shell elevated, slit very narrow, on the anterior margin (in subg. Rimula, it is between the apex and the margin), radula bilaterally asymmetrical; Subemarginula, margin indented by a shallow groove; Scutus (= Parmophorus) shell oblong, depressed, nicked in front, largely covered by the mantle.
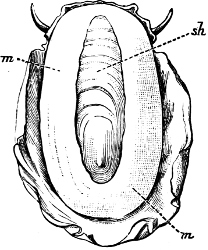
Fig. 268.—Scutus australis Lam., Australia: m, m, mantle; sh, shell, × ⅔.
Fam. 2. Haliotidae.—Right ctenidium the smaller, epipodial line broad, profusely lobed; shell rather flattened, spire short, last whorl very large, with a row of perforations on the left side,[407] which become successively obliterated; through these holes, the posterior of which is anal, pass tentacular appendages of the mantle; no operculum. Cretaceous——. Single genus, Haliotis; principal sub-genera Padollus, Teinotis.
Fam. 3. Pleurotomariidae.—Central tooth single, narrow, about 26 laterals, 60 to 70 uncini. Shell generally variously trochiform, nacreous, operculate, with a rather broad marginal sinus in the last whorl; as this sinus closes up it forms an “anal fasciole” or “sinus band.” Cambrian——. Principal genera: Scissurella, epipodial line with several long ciliated appendages at each side, shell very small, slit open, sinus band extending nearly to apex; Schismope, anal slit closed in the adult into an oblong perforation; Murchisonia (Palaeozoic only), shell long, turreted, whorls angulate or keeled with a sinus band; Odontomaria (Palaeozoic only), shell tubular, curved; Polytremaria (Carboniferous), shell turbinate, slit a series of small holes connected by a passage; Trochotoma, shell trochiform, perforation consisting of two narrow holes united by a slit; Pleurotomaria, branchiae almost symmetrical, radula as above, shell variously spiral.
In Pleurotomaria we have the case of a genus long supposed to be extinct. More than 1100 fossil species have been described, and within the last 38 years about 20 specimens, belonging to 5 species, have been discovered in a living state.
Fig. 269.—Pleurotomaria adansoniana Cr. and F., Tobago. × ½.
Fam. 4. Bellerophontidae.—Shell nautiloid, spire generally concealed, aperture large, sinus or perforations central (Fig. 179, p. 266). Ordovician—Trias. Genera: Bellerophon, Trematonotus, Cyrtolites.
Section II. Azygobranchiata.—One ctenidium (the left) present.
[408]
Fam. 1. Cocculinidae.—A single cervical ctenidium, foot broad, no eyes, shell patelliform, with caducous spire. Single genus, Cocculina. Deep water.
Fam. 2. Stomatellidae.—A single (left) ctenidium, front third free, shell nacreous, spiral or patelliform, depressed, last whorl large. Jurassic——. Genera: Stomatella (subg. Synaptocochlea, Niphonia), shell depressed, spirally ribbed, spire short, operculum present; Phaneta, fluviatile only, shell trochiform, imperforate, last whorl keeled, sinuate in front; Stomatia, spire short, surface tubercled or keeled, no operculum; Gena, shell haliotis-shaped, surface smooth, aperture very large; Broderipia, shell patelliform, spiral apex often lost.
Fam. 3. Cyclostrematidae.—Tentacles ciliated, thread-like, snout bilobed, foot truncated in front, angles produced into a filament, shell depressed, umbilicated, not nacreous. Eocene——. Principal genera: Cyclostrema, Teinostoma, Vitrinella.
Fam. 4. Liotiidae.—Epipodial line with a lobe behind each eye-peduncle, shell solid, trochiform, longitudinally ribbed or trellised, aperture round, operculum multispiral, hispid, corneous, with a calcareous layer. Silurian——. Principal genera: Liotia, Craspedostoma (Silurian), Crossostoma (Jurassic).
Fig. 270.—Monodonta canalifera Lam., New Ireland. (After Quoy and Gaimard.)
Fam. 5. Trochidae.—Snout short, broad, frontal lobes often present, epipodial line furnished with cirrhi; shell nacreous, variously spiral, operculum corneous, multispiral, nucleus central (Fig. 182, p. 268). Silurian——. (i.) Trochinae.—Frontal lobes present, lateral teeth (= side centrals) 5 only, no jaws, peristome incomplete. Principal genera: Trochus (subg. Cardinalia, Tectus, Infundibulum, Clanculus), Monodonta (subg. Diloma), Cantharidus (subg. Bankivia, Thalotia), Gaza (subg. Microgaza), Callogaza, Bembix, Chlorostoma. (ii.) Gibbulinae.—Frontal lobes and jaws present, laterals often more than 5, peristome incomplete. Principal genera: Gibbula (subg. Monilia, Aphanotrochus, Enida), Minolia, Circulus, Trochiscus, Livona, Photinula, Margarita, Solariella, Calliostoma, Turcica, Basilissa, Euchelus (subg. Olivia, Perrinia). (iii.) Delphinulinae.—No frontal lobes, jaws present; shell solid,[409] surface spirally lirate, scaly, spinose, umbilicate, peristome continuous. Single genus, Delphinula. (iv.) Umboniinae.—Eyes pedunculate, left tentacle attached to a frontal appendage, mantle reflected over edge of aperture, lateral teeth 6 on each side; shell polished, peristome incomplete, umbilicus generally closed by a callosity. Principal genera: Umbonium, Ethalia, Isanda, Camitia, Umbonella, Chrysostoma.

Fig. 271.—Phasianella australis Gmel., Australia.
Fam. 6. Turbinidae.—Epipodial line with slender cirrhi, snout broad, short, eyes pedunculate at outer base of tentacles, a frontal veil between tentacles; shell turbinate, solid, aperture continuous, operculum solid, calcareous, usually paucispiral, convex exteriorly (Fig. 182, p. 268). Silurian——. (i.) Phasianellinae.—Shell bulimoid, polished, not nacreous, coloured in patterns, aperture oval. Single genus, Phasianella (Fig. 271). (ii.) Turbininae.—Shell very solid, nacreous within, aperture circular or long oval. Principal genera, Turbo, whorls rounded above and below, spines, if present, becoming more prominent with age, operculum smooth or granulose, nucleus sub-central; subg. Callopoma, Ninella, Marmorostoma, Sarmaticus, Prisogaster; Astralium, whorls flattened above and below, spines, if present, becoming less prominent with age, operculum oblong, often excavated at centre, last whorl large, nucleus marginal or sub-marginal; subg. Lithopoma, Imperator, Guildfordia, Bolma, Cyclocantha, Uvanilla, Cookia, Pomaulax, Pachypoma. (iii.) Cyclonematinae.—Shell nacreous, umbilicate, operculum conical outside, whorls scalariform. Principal genera: Cyclonema, Horiostoma (?), Amberleya (Silurian to Lias). (iv.) Leptothyrinae.—Shell small, solid, depressed, operculum nearly flat, nucleus sub-central. Genera: Leptothyra, Collonia (?).
Fam. 7. Neritopsidae.—Tentacles wide apart, long, eyes on short peduncles at the outer base; shell solid, neritiform or naticoid, aperture semi-lunar or oval; operculum (Fig. 183, p. 269) thick, calcareous, non-spiral, exterior face smooth, interior face divided into two unequal parts, with a broad median appendage. Devonian——. Principal genera: Neritopsis (one recent species), Naticopsis (Devonian to Miocene).
[410]
Fam. 8. Macluritidae.—Shell discoidal, whorls few, longitudinally grooved behind, right side convex, deeply umbilicated, left side flat; operculum very thick, nucleus excentrical, internal face with two apophyses, one very large. The general appearance is more that of an inequivalve bivalve, such as Requienia, than of a spiral gasteropod. Palaeozoic——. Single genus, Maclurea.
Fam. 9. Neritidae.—Snout short, tentacles long, eyes pedunculate at their outer base, branchia triangular, free at the front end, epipodium without cirrhi, penis near the right tentacle; shell solid, imperforate, turbinate to almost patelliform, spire short, internal partitions absorbed (p. 168), columellar region broad, edge simple or dentate, operculum calcareous, spiral or non-spiral, with prominent apophyses on the interior face, one of which locks behind the columellar lip. Jurassic——. Principal genera: Nerita (Fig. 13, p. 17); Neritina (chiefly brackish water and fluviatile), sub-genus Clithon, usually coronated with spines; Velates (Tertiary), Neritoma (Jurassic), Deianira (Cretaceous), Septaria (= Navicella), shell more or less narrowly patelliform, with terminal apex, aperture very large, with a broad columellar septum, operculum too small for the aperture, more or less covered by the integument of the foot; fluviatile only; Pileolus (Jurassic to Cretaceous).
Fam. 10. Hydrocenidae.—Branchia replaced by a pulmonary chamber, eyes at the outer base of the tentacles, marginals of the radula very oblique, centrals often wanting; shell small, conical, whorls convex, operculum calcareous, with a prominent apophysis. Recent. Principal genera: Hydrocena, Georissa.
Fam. 11. Helicinidae.—Branchia replaced by a pulmonary chamber, heart with one auricle; shell globular, with a short spire, internal partitions absorbed; operculum without apophysis. Carboniferous——. Principal genera: Helicina (Fig. 18B, p. 21; subg. Alcadia, Schasicheila, Heudeia, Calybium), Eutrochatella (subg. Lucidella), Stoastoma, Bourcieria, Dawsonella (Carboniferous).
Fam. 12. Proserpinidae.—Branchia replaced by a pulmonary chamber, mantle partly reflected over the shell, eyes sessile; shell depressed, discoidal, columella folded or truncated at the base, whorls with one or more internal plicae, internal partitions absorbed, no operculum. Eocene——. Single genus; Proserpina, subg. Proserpinella, Cyane, Dimorphoptychia (Eocene), and Ceres (Fig. 18C, p. 21).
[411]
Sub-order II. Monotocardia.—Heart with one auricle, one ctenidium (the left), monopectinate, fused with the mantle (except in Valvata), one kidney, not receiving the genital products, nervous system somewhat concentrated, proboscis and penis usually present.
(a) Ptenoglossa.—Radula with formula ∞. ᴑ. ∞, teeth similar throughout, outermost largest (p. 224).
Fam. 1. Ianthinidae.—Snout prominent, blunt, no eyes, shell helicoid, fragile, bluish, no operculum; eggs carried on a raft of vesicles attached to the foot (Fig. 42, p. 126). Pelagic only. Pliocene ——. Genera: Ianthina, Recluzia.
Fam. 2. Scalariidae.—Shell long, turriculate, whorls often partly uncoiled, with longitudinal ribs and prominent lamellae, aperture circular, operculum spiral, corneous, animal carnivorous. Ordovician ——. Principal genera: Scalaria, Eglisia, Elasmoneura (Silurian), Holopella (Silurian to Trias), Aclis.
(b) Taenioglossa.—Radula with normal formula 2.1.1.1.2, marginals sometimes multiplied (p. 223).
Section I. Platypoda.—Foot more or less flattened ventrally.
Fam. 1. Naticidae.—Foot very large, produced before and behind, propodium reflected upon the head, eyes absent or buried in the integument, central and lateral tooth of the radula tricuspid, middle cusp strong; shell globular or auriform, outer lip simple, operculum corneous or calcareous, nucleus excentrical. Carboniferous ——. Principal genera: Natica, with many sub-genera; Ampullina (Tertiary); Amaura; Deshayesia (Tertiary); Sigaretus (Fig. 91, p. 186), shell auriform, last whorl very large, operculum much too small for the aperture.
Fam. 2. Lamellariidae.—Mantle reflected over more or less of the shell, shell delicate, no operculum. Eocene——. Principal genera: Lamellaria, shell completely internal, transparent, auriform; some species deposit their eggs on compound Ascidians (p. 74); Velutina, shell almost entirely external, paucispiral, with a thick periostracum; Marsenina, shell auriform, partly internal; Onchidiopsis, shell a membranous plate, internal.
Fam. 3. Trichotropidae.—Branchial siphon short, eyes on the outer side of the tentacles; radula closely allied to that of Velutina; shell conical, last whorl rather large, periostracum thick and hairy, operculum blunt claw-shaped, nucleus terminal. Cretaceous——. Genera: Trichotropis, Torellia.
[412]
Fam. 4. Naricidae.—Tentacles broad in the middle, with sessile eyes at the exterior base, propodium narrow, quadrangular, a large epipodial veil on each side of the foot; shell naticoid, cancellated, with velvety periostracum. Jurassic——. Single genus: Narica.
Fam. 5. Xenophoridae.—Foot divided by a groove, anterior portion the larger; central tooth heart-shaped, with blunt cusps, lateral large, roughly triangular, marginals long, falciform; shell trochiform, somewhat flattened, attaching various fragments externally. Devonian——. Single genus, Xenophora (Figs. 25, 26, p. 64).
Fam. 6. Capulidae.—Ctenidium deeply and finely pectinate, visceral sac scarcely spiral, penis long, behind the right tentacle; shell roughly patelliform, with scarcely any spire, interior polished, usually with a septum or internal plate of variable form, no operculum. Devonian——. Principal genera (Fig. 155, p. 248); Capulus, shell cap-shaped, no internal plate; Platyceras (Palaeozoic, see p. 76), Diaphorostoma (Palaeozoic), Addisonia (?); Crucibulum, internal appendage funnel-shaped; Crepidula (including Crepipatella and Ergaea), shell slipper-shaped, with a large septum; Calyptraea (including Galerus and Trochita), internal lamina semi-spiral.
Fam. 7. Hipponycidae.—Foot aborted, animal sedentary, adductor-muscle shaped like a horse’s hoof, fastened on the ventral side to the region of attachment, or to a thin calcareous plate which closes the aperture like a valve; ventral side of the body surrounded by a mantle with papillose border, which corresponds morphologically to the epipodia, head emerging between the dorsal and ventral mantles. Shell thick, bluntly conical, surface rugose. Eocene ——. Genera: Hipponyx; Mitrularia, a narrow half funnel-shaped appendage within the shell.
Fig. 272.—Two specimens of Crepidula (marked a and b) on an old shell of Murex radix Gmel.
Fam. 8. Solariidae.—Foot large, eyes sessile, near the outer base of the tentacles, radula abnormal (p. 224); shell more or less depressed, lip simple, umbilicus wide, margins often crenulated, operculum variable. The proper position of the family is quite uncertain. Ordovician——. (i.) Solariinae. Genera: Solarium,[413] shell depressed, highly finished, angular at periphery, operculum corneous, central tooth absent, laterals and marginals numerous, long, and narrow; Platyschisma (Silurian). (ii.) Toriniinae. Genera: Torinia, whorls usually rounded, operculum (Fig. 183) conically elevated, spiral externally, central tooth present, marginals few, edge pectinated; Omalaxis. (iii.) Euomphalinae, shell planorbiform, whorls rounded. Genera: Euomphalus, Ophileta, Schizostoma, Eccyliomphalus (all Palaeozoic).
Fam. 9. Homalogyridae.—Tentacles absent, eyes sessile, central tooth unicuspid on a quadrangular base, laterals and marginals replaced by an oblong plate; shell very small, planorbiform. Recent. Single genus: Homalogyra, whose true position is uncertain.
Fig. 273.—Solarium perspectivum Lam., Eastern Seas.
Fam. 10. Littorinidae.—Proboscis short, broad, tentacles long, eyes at their outer bases, penis behind the right tentacle; reproduction oviparous or ovoviviparous, radula very long; shell turbinate, solid, columella thickened, lip simple, operculum corneous, nucleus excentrical. Jurassic——. Principal genera: Littorina (radula, Fig. 16, p. 20), Cremnoconchus (p. 16), Fossarina; Tectarius, shell tubercled or spinose; Risella, base slightly concave; Lacuna, shell thin, grooved behind the columellar lip.
Fam. 11. Fossaridae.—Shell turbinate, solid, small, white, spirally ribbed, outer lip simple. Miocene——. Principal genus, Fossarus.
Fam. 12. Cyclophoridae.—Ctenidium replaced by a pulmonary sac, tentacles long, thread-like (radula, Fig. 17, p. 21); shell variously spiral, peristome round, often reflected, operculum circular. Terrestrial only. Cretaceous——. (i.) Pomatiasinae, shell high, conical, longitudinally striated, operculum consisting of two laminae united together. Single genus, Pomatias. (ii.) Diplommatininae, shell more or less pupiform, peristome thickened or reflected, often double. Genera: Diplommatina (subg., Nicida, Palaina, Paxillus, Arinia), shell dextral or sinistral, small, columella often denticulated; Opisthostoma (Fig. 208, p.[414] 309), last whorl disconnected, often reflected back upon the spire. (iii.) Pupininae, shell more or less lustrous, bluntly conical, lip with a channel above or below. Genera: Pupina (subg., Registoma, Callia, Streptaulus, Pupinella, Anaulus), Hybocystis (Fig. 205, p. 305), Cataulus, Coptochilus, Megalomastoma. (iv.) Cyclophorinae, shell turbinate or depressed, operculum corneous or calcareous. Genera: Alycaeus, Craspedopoma, Leptopoma, Lagochilus, Cyclophorus (Fig. 206, p. 306); including Diadema, Aulopoma, Ditropis, and others), Aperostoma (including Cyrtotoma and others), Cyathopoma, Pterocyclus (subg., Myxostoma, Spiraculum, Opisthoporus, and Rhiostoma (Fig. 180, p. 266), Cyclotus, Cyclosurus, and Strophostoma.
Fam. 13. Cyclostomatidae.—Ctenidium replaced by a pulmonary sac, tentacles obtuse, foot with a deep longitudinal median groove; central tooth, lateral, and first marginal more or less bluntly cusped, second marginal large, edge pectinate; shell variously spiral, spire usually elevated, aperture not quite circular; operculum generally with an external calcareous and an internal cartilaginoid lamina, rarely corneous. Terrestrial only. Cretaceous——. Genera: Cyclostoma (subg., Leonia, Tropidophora, Rochebrunia, Georgia, Otopoma, Lithidion, Revoilia), Cyclotopsis, Choanopoma (subg., Licina, Jamaicia, Ctenopoma, Diplopoma, Adamsiella), Cistula (subg., Chondropoma, Tudora), Omphalotropis (subg., Realia, Cyclomorpha), Hainesia, Acroptychia.
Fig. 274.—Cyclostoma campanulatum Pfr., Madagascar.
Fam. 14. Aciculidae.—Ctenidium replaced by a pulmonary sac, tentacles cylindrical, pointed at the end, eyes behind their base, foot long and narrow; central tooth and lateral very similar, pinched in at the sides, external marginal broad, edge finely pectinate; shell small, acuminate, with a blunt spire, operculum corneous. Terrestrial only. Tertiary——. Genus, Acicula (= Acme).
Fam. 15. Truncatellidae.—Ctenidium replaced by a pulmonary sac, proboscis very long, eyes sessile, behind the base of the tentacles, shell small, evenly cylindrical, apex truncated in the adult. Eocene——. Genera: Truncatella (subg., Taheitia, Blanfordia, and Tomichia), Geomelania (subg., Chittya and Blandiella), Cecina (?).
[415]
Fam. 16. Rissoidae.—Eyes at the external base of the tentacles, epipodium with filaments, operculigerous lobe with appendages; central tooth pleated at the basal angles, lateral large, bluntly multicuspid, marginals long, narrow, denticulate at the edge; shell small, acuminate, often elaborately sculptured, mouth entire or with a shallow canal, operculum corneous. Marine or brackish water. Jurassic——. Principal genera: Rissoa (subg., Folinia, Onoba, Alvania, Cingula, Nodulus, Anabathron, Fenella, Iravadia, and others), Scaliola (shell agglutinating fragments of sand, etc.), Rissoina (lip thickened, operculum with an apophysis as in Nerita), Barleeia, Paryphostoma (Eocene).
Fam. 17. Hydrobiidae.—Eyes at the outer base of the tentacles, penis behind the right tentacle, prominent, operculigerous lobe without filaments; radula rissoidan, central tooth often with basal denticulations; shell more or less acuminate, small, aperture entire, operculum corneous or calcareous. Brackish or fresh water. Jurassic——. Principal genera: Baicalia, with its various sub-genera (p. 290); Pomatiopsis, Hydrobia, Bithynella, Micropyrgus (Tertiary), Pyrgula, Emmericia, Benedictia, Lithoglyphus, Tanganyicia, Limnotrochus (?), Jullienia, Pachydrobia, Potamopyrgus, Littorinida, Amnicola, Fluminicola (subg., Gillia, Somatogyrus), Bithynia, Fossarulus (Tertiary), Stenothyra.
Fam. 18. Assimineidae.—Ctenidium replaced by a pulmonary sac, no true tentacles, eye-peduncles long, retractile; radula that of Hydrobia; shell small, conoidal, operculum corneous, nucleus sub-lateral. Eocene——. Genera: Assiminea, Acmella.
Fam. 19. Skeneidae.—Radula resembling that of Hydrobia; shell very small, depressed, widely umbilicated, operculum corneous. Pleistocene——. Single genus, Skenea.
Fam. 20. Jeffreysiidae.—Mantle with two pointed ciliated appendages in front, tentacles ciliated, eyes sessile, far behind the base of the tentacles; marginal teeth sometimes absent; shell small, thin, pellucid, whorls rather swollen, operculum with marginal nucleus, divided by a rib on the inner face. Recent. Genera: Jeffreysia, Dardania. Marine, living on algae.
Fam. 21. Litiopidae.—Epipodium with cirrhi on each side, operculigerous lobe with appendages; radula rissoidan; shell small, conical, columella truncated, operculum corneous. Eocene——. Genera: Litiopa, living on the Sargasso weed, suspended by a long filament; Alaba, Diala.
[416]
Fam. 22. Adeorbidae.—Radula essentially rissoidan; shell depressed, circular or auriform, widely umbilicated, operculum corneous, paucispiral, nucleus excentrical. Pliocene ——. Principal genera: Adeorbis, Stenotis, Megalomphalus.
Fam. 23. Viviparidae.—Snout blunt, tentacles long, right tentacle in the male deformed, pierced with a hole corresponding to the aperture of the penis, two cervical lobes, the right being siphonal, foot with an anterior transverse groove; teeth broad, shallowly pectinate at the ends; shell turbinate, whorls more or less rounded, aperture continuous, operculum corneous, nucleus sub-lateral, with a false sub-central nucleus on the external face. Animal ovoviviparous. Fresh-water. Cretaceous ——. Genera: Vivipara (= Paludina), subg., Cleopatra, Melantho, Tulotoma; Tylopoma (Tertiary), and Lioplax.
Fam. 24. Valvatidae.—Branchia exserted, bipectinate, carried on the back of the neck, a filiform appendage (Fig. 66, p. 159) on the right of the neck, penis under the right tentacle, prominent, eyes sessile, behind the tentacles; radula like that of Vivipara; shell small, turbinate or flattened, operculum corneous, nucleus central. Fresh water. Jurassic ——. Single genus, Valvata.
Fam. 25. Ampullariidae.—Snout with two tentacles, tentacles proper very long, tapering, eyes prominently pedunculate, two cervical lobes, the left siphonal, respiratory cavity divided by a partition, a large branchia in the right chamber, the left functioning as a pulmonary sac (Fig. 65, p. 158); radula large, central tooth multicuspid, base broad, lateral and marginals falciform, simple or bicuspid; shell large, turbinate or flattened, spire small, whorls rounded; operculum generally corneous, nucleus sub-lateral, false nucleus as in Vivipara. Fresh water. Cretaceous ——. Single genus, Ampullaria (subg., Ceratodes, Pachylabra, Asolene, Lanistes, and Meladomus).
Fam. 26. Cerithiidae.—Branchial siphon present, short, eyes variable in position; central tooth small, evenly cusped, lateral hollowed at base, multicuspid, marginals narrow; shell long, turriculate, whorls many, generally tuberculate, varicose or spiny, aperture sometimes strongly channelled; operculum corneous, subcircular, nucleus nearly central. Marine or brackish water. Trias ——. Principal genera: Triforis, shell small, generally sinistral; Fastigiella, Cerithium (Fig. 12, p. 16), Bittium, Potamides (subg., Tympanotomus, Pyrazus, Pirenella, Telescopium, Cerithidea, Lampania, all brackish water), Diastoma[417] (Eocene), Cerithiopsis; Ceritella (Jurassic), Brachytrema (Jurassic), and Planaxis (subg., Quoyia and Holcostoma).
Fam. 27. Modulidae.—No siphon, radula of Cerithium; shell with short spire, columella strongly toothed at the base, aperture nearly circular. Recent. Single genus, Modulus.
Fam. 28. Nerineidae.—Shell solid, long, sub-cylindrical, aperture channelled, columella and interior of whorls with continuous ridges, extending up the spire. Genera: Nerinea (Trias to Cretaceous), Aptyxiella (Jurassic).
Fam. 29. Melaniidae.—Border of mantle festooned, foot broad, with an anterior groove, penis present; radula closely resembling that of Cerithium; shell long, spiral, with a thick periostracum, surface with tubercles, ribs, or striae, suture shallow; operculum corneous, paucispiral, nucleus excentrical. Animal ovoviviparous. Fresh water. Cretaceous ——. Principal genera: Melania (with many sections or sub-genera), Pachychilus, Claviger (= Vibex), Hemisinus, Pirena, Melanopsis, Tiphobia, Paludomus (subg., Philopotamis, Tanalia, Stomatodon), Hantkenia (Eocene), Larina (?).
Fam. 30. Pleuroceridae.—Mantle edge not festooned, no copulatory organ, otherwise like Melaniidae; operculum with nucleus sub-marginal. Animal oviparous. Fresh-water. Cretaceous ——. Genera: Pleurocera (including Io, Fig. 12, p. 16, Angitrema, Lithasia, Strephobasis), Goniobasis, Anculotus, Gyrotoma.
Fam. 31. Pseudomelaniidae.—Shell resembling that of Melaniidae, but marine. Genera: Pseudomelania, Loxonema, Bourguetia, Macrochilus. Palaeozoic to Tertiary strata.

Fig. 275.—Melania confusa Dohrn, Ceylon.
Fam. 32. Turritellidae.—Mantle with a siphonal fold on the right side; radula variable (p. 224); shell long, whorls many, slowly increasing in size, transversely ribbed or striated, aperture small; operculum corneous, nucleus central. Jurassic ——. Principal genera: Turritella, Mesalia, Protoma, Mathilda (?).
Fam. 33. Coecidae.—Tentacles long, eyes sessile at their base; shell small, spiral in the young form, spire generally lost in the adult, the shell becoming simply a straight or curved cylinder; operculum corneous, multispiral. Eocene ——. Single genus, Coecum.
[418]
Fam. 34. Vermetidae.—Visceral sac greatly produced, irregularly spiral, no copulatory organs (radula, Fig. 126, p. 223), shell tubular, irregularly coiled, last whorls often free, aperture circular; operculum corneous, circular, nucleus central. Carboniferous ——. Principal genera: Vermetus; Siliquaria (Fig 153, p. 248), a long fissure, or series of holes, runs along a considerable part of the shell, operculum with outer face spiral, elevated.
Fam. 35. Strombidae.—Foot narrow, arched, metapodium greatly produced, snout long, eye peduncles long, thick, eyes elaborate, siphon short, penis prominent, bifurcate; central tooth with strong median cusp, marginals falciform, slender, edge more or less denticulate; shell solid, spire conical, outer lip generally dilated into wings or digitations, channelled before and behind, a labial sinus at the base, distinct from the anterior canal; operculum small for the aperture, corneous, claw-shaped, edge notched. Lias ——. Genera: Strombus (Fig. 99, p. 200); Pereiraea (Miocene), Pteroceras (Fig. 277; digitations of the outer lip very strong), Rostellaria (spire produced, anterior canal very long), Rimella, Pterodonta, Terebellum (base of shell truncate, spire short).
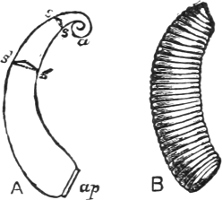
Fig. 276.—Development of Coecum: A, showing the gradual formation of septa; a, apex; ap, aperture; ss, first septum; s´s´, second septum. (After de Folin.) B, adult form of C. eburneum Ad., Panama, x 10.
Fam. 36. Chenopodidae (= Aporrhaidae).—Foot flat; lateral and marginal teeth not denticulate; shell resembling that of Strombus, outer lip dilated, wing-like, no labial sinus. Jurassic ——. Genera: Chenopus (= Aporrhais, Diastema, Malaptera, Harpagodes, Alaria) (last four from Secondary strata).
Fam. 37. Struthiolariidae.—Radula allied to that of Strombus, marginals occasionally multiplied; shell buccinoid, very solid, outer lip thickened, canal short, operculum claw-shaped, notched, nucleus terminal. Tertiary ——. Single genus, Struthiolaria (subg., Perissodonta, marginal teeth multiplied).
Fam. 38. Cypraeidae.—Mantle with two large lateral lobes reflected and meeting over the shell, siphon small; central and lateral teeth bluntly tricuspid or multicuspid, laterals fairly broad, edges cusped or finely pectinate; shell polished, solid, spire generally concealed in the adult or overlaid with enamel, aperture straight, narrow, nearly as long as the shell, toothed at[419] the sides, channelled at each end, labium inflected; no operculum. Jurassic ——. Genera: Ovula (including Amphiperas, Transovula, Cyphoma, Radius, Simnia), Pedicularia, Cypraea (with subg., Cypraeovula, Cypraedia, and Trivia), and Erato.
Fam. 39. Doliidae.—Foot expanded, wider and longer than the shell, truncated and thickened in front, siphon very long and narrow; central tooth with very strong median and small lateral and basal cusps, lateral and marginals bluntly falciform; shell ventricose, without varices, spire short, outer lip generally simple, anterior canal rather wide, no operculum. Cretaceous ——. Genera: Dolium (subg., Malea, outer lip thickened, denticulate, reflected); Pirula, mantle with two lateral lobes reflected over part of the shell, shell fig-shaped (Fig. 278).
Fig. 277.—Three stages in the growth of Pteroceras rugosum Sowb., E. Indies, showing the development of the ‘fingers.’
Fam. 40. Cassididae.—Foot broad, siphon long (radula, Fig. 125, p. 223); shell ventricose, with varices, spire short, outer lip reflected or thickened, anterior canal short, recurved narrow; operculum semi-lunar, with ribs radiating from a marginal[420] nucleus. Cretaceous ——. Genera: Cassis (subg., Semicassis and Cypraecassis), Morio (= Cassidaria), Oniscia.
Fam. 41. Columbellinidae.—Shell solid, ribbed, usually cancellated, with an oblique posterior canal, columella callous, more or less reflected. Genera: Columbellina, Columbellaria, Zittelia, Petersia, Alariopsis (?). Secondary strata only.
Fam. 42. Tritonidae.—Foot short, narrow; siphon short, not prominent; radula allied to that of Cassididae; shell thick, varicose; outer lip inflected and thickened, canal long, periostracum often thick and hairy, operculum corneous, nucleus terminal or sub-marginal. Cretaceous ——. Genera: Triton (Fig. 191, p. 275; subg., Epidromus, Plesiotriton, Simpulum, Ranularia, Argobuccinum); Persona, aperture toothed, narrow; columella reflected upon the last whorl; Ranella, shell dorso-ventrally compressed, generally with two continuous lateral varices, posterior canal present.
The position of the following four families is doubtful:—
Fam. 43. Oocorythidae.—Siphon short, foot broad, eyes absent, radula taenioglossate; shell buccinoid or cassidiform, operculum corneous, spiral. Recent. Single genus, Oocorys.
Fam. 44. Subulitidae.—Shell elongate, fusiform, smooth; suture shallow, base truncate or rounded, aperture channelled or notched. Ordovician to Trias. Genera: Subulites, Fusispira, Euchrysallis.
Fig. 278.—Pirula Dussumieri Val., Philippines. × ½.
Fam. 45. Seguenziidae.—Radula taenioglossate, shell trochiform, aperture channelled, columella twisted, operculum multispiral, nucleus central. Pliocene ——. Single genus, Seguenzia.
Fam. 46. Choristidae.—Anterior tentacles united by a frontal veil, posterior simple; eyes absent, foot with tentaculae before and behind; three central teeth, outer marginal with a basal plate; shell helicoid, suture deep, peristome continuous, operculum corneous, paucispiral. Pliocene ——. Single genus, Choristes.
Section II. Heteropoda.—Foot fin-shaped, not flat.
The Heteropoda are free-swimming Mollusca, being, like the Pteropoda, Gasteropoda modified to suit their pelagic environment. Their nervous system is streptoneurous, and they are[421] therefore probably derived from the Prosobranchiata, but they are highly specialised forms. Pelseneer considers them far more widely removed from the Streptoneura than the Pteropoda are from the Euthyneura. They swim on the surface “upside down,” i.e. with the ventral side uppermost.
The tissues and shell are transparent, permitting observation of the internal organs. In the Pterotrachaeidae the foot takes the form of a fan-shaped disc, usually furnished with a sucker. The body is compressed at the posterior end, often with a ventral “fin.” In Atlanta the foot consists of three very distinct parts: a propodium, a mesopodium, on which is a small sucker, and a metapodium, which carries the operculum. The branchiae are carried on the visceral sac, and are free in Pterotrachaea, slightly protected by the shell in Carinaria, and entirely covered in Atlanta; absent altogether in Firoloida.
The head carries two tentacles (except in Pterotrachaea), with large, highly organised eyes on short lobes at their outer base. The alimentary tract consists of a long protrusible proboscis, with a taenioglossate radula (Fig. 132, p. 227), a long oesophagus, and a slightly flexured intestine. In Atlanta the visceral sac is spiral and protected by a spiral planorbiform shell; in Carinaria the visceral sac is small, conical, protected by a very thin capuliform shell. There is no shell in Pterotrachaea or Firoloida.
The Heteropoda are dioecious. In the male there is a flagellum behind the penis, which is near the middle of the right side. Pterotrachaea lays long chains of granular eggs, and has been noticed to produce a metre’s length in a day. The eggs of Atlanta are isolated. The embryo has a deeply bilobed velum.
Fam. 1. Pterotrachaeidae.—Body long, with a caudal “fin;” branchiae dorsal, free or partly protected by a shell; foot consisting of a muscular disc, with or without a sucker.
Pterotrachaea proper has no mantle, shell, or tentacles. The branchiae are disposed round the visceral sac, at the upper part of which is the anus. In Firoloida the body is abruptly truncated behind, with a long filiform segmented caudal appendage; visceral sac at the posterior end: fin-sucker present or absent in both male and female. Cardiapoda resembles Carinaria, but the visceral sac is more posterior and is only slightly protected by[422] a very small spiral shell. Carinaria (Fig. 279) has a rugose translucent skin, visceral sac sub-median, apparently pedunculated, covered by a capuliform shell. The larval shell, which persists in the adult, is helicoid.
Fam. 2. Atlantidae.—Shell spiral, operculate, covering the animal. Branchiae in a dorsal cavity of the mantle; foot trilobed, with a small sucker on the mesopodium.
The shell of Atlanta is discoidal and sharply keeled, while that of Oxygyrus is nautiloid, with the spire concealed, no keel, aperture dilated.
(c) Gymnoglossa.—Radula and jaws absent; proboscis prominent, sexes probably separate, penis present. The section is probably artificial and unnecessary, the families composing it being, in all probability, Taenioglossa which have lost their radula in consequence of changed conditions of life (pp. 79, 225).
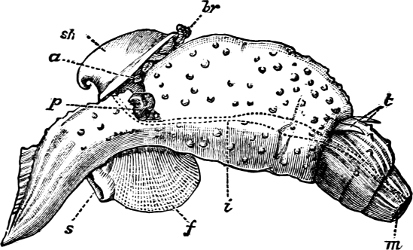
Fig. 279.—Carinaria mediterranea Lam., Naples: a, anus; br, branchiae; f, foot; i, intestine; m, mouth; p, penis; s, sucker; sh, shell; t, tentacles. × ½.
Fam. 1. Eulimidae.—Proboscis very long, retractile, mantle forming a siphonal fold; shell small, long, subulate, polished; suture shallow, aperture continuous, operculum present or absent. Animal often parasitic, sucking the juices of its host by its long proboscis. Trias——. Genera: Eulima (subg., Subularia, Arcuella, Apicalia, Mucronalia, Stiliferina, and others), Stilifer, Scalenostoma, Niso, and Hoplopteron.
Fam. 2. Pyramidellidae.—Tentacles auriform, proboscis as in Eulimidae, a prominent mentum or flap under the buccal orifice; shell usually small, conical; suture shallow, apical whorls (the embryonic shell) sinistral (p. 250), operculum corneous, paucispiral; nucleus excentrical. Trias——. Genera: Pyramidella (subg., Syrnola, Otopleura, Chrysallida, Mumiola), Odostomia, Eulimella, Murchisoniella, Turbonilla (subg., Dunkeria and Cingulina).
(d) Rachiglossa (p. 220).—Proboscis long, retractile; siphon distinct, radula without uncini, sometimes without laterals; teeth strongly cusped; shell generally wholly external.
[423]
Fam. 1. Muricidae.—Eyes sessile at the outer base of the tentacles, penis large, behind the right tentacle, radula within the retractile proboscis, central tooth (Fig. 119, p. 220) with at least three strong cusps, laterals plain; shell solid, more or less tuberculate, spiny and varicose, anterior canal varying from a mere notch to a long channel. Cretaceous——. Principal genera: (i.) Muricinae, nucleus of operculum sub-terminal; Trophon, Typhis, Murex (with many subdivisions), Ocinebra (including Cerastoma, Vitularia, and Hadriania), Urosalpinx, Eupleura, Pseudomurea. (ii.) Purpurinae, nucleus of operculum lateral; Rapana (including Latiaxis), Purpura (with subg., Cuma, Iopas, Vexilla, and Pinaxia), Monoceros (including Chorus), Purpuroidea (Secondary strata), Pentadactylus, Sistrum, Concholepas.
Fam. 2. Coralliophilidae.—Animal living in Madrepores, resembling Purpura, radula absent; shell variously shaped, often deformed or tubular, operculum that of Purpura, if present. Miocene——. Principal genera: Rhizochilus, Coralliophila, Leptoconchus, Magilus (Fig. 29, p. 75), Rapa.
Fam. 3. Columbellidae.—(Radula, Fig. 123, p. 222.) Shell small, solid, fusiform, aperture narrow, canal short, outer lip thickened. Miocene——. Single genus, Columbella (subg., Nitidella, Anachis, Meta, Strombina, Atilia, Conidea, Amphissa, Mitrella, and others).
Fam. 4. Nassidae.—Foot long and broad, often with terminal appendages; siphon long, eyes on outer base of tentacles, central tooth of radula arched, multicuspid, lateral strongly bicuspid, with small denticles between the cusps; shell rather small, buccinoid, columella more or less callous, outer lip thickened, often toothed; operculum corneous, edges often toothed. Miocene——. Principal genera: Nassa (with many sections), Amycla, Desmoulea, Cyclonassa, Canidia (subg., Clea and Nassodonta), Dorsanum, Bullia (= Buccinanops, Fig. 62, p. 185), Truncaria.
Fam. 5. Buccinidae.—Siphon rather long, eyes at outer base of tentacles; central tooth of radula with 5 to 7 cusps, laterals bicuspid or tricuspid (Fig. 118, p. 220); shell more or less fusiform, thick, covered with a periostracum, canal of varying length, outer lip simple or thickened; operculum corneous, nucleus variable in position. Cretaceous——. Principal genera: Group i. Chrysodomus (with sections Neptunea, Volutopsis, Pyrolofusus,[424] Jumala), subg., Sipho; Siphonalia (subg., Kelletia). Group ii. Liomesus (= Buccinopsis). Group iii. Buccinum (Fig. 1 B, p. 6; subg., Volutharpa, Neobuccinum). Group iv. Cominella, Tritonidea, Pisania, Euthria; Anura (Miocene), Genea (Pliocene), Metula, Engina. Group v. Phos, Hindsia. Group vi. Dipsaccus (= Eburna), Macron. Group vii. Pseudoliva.
Fam. 6. Turbinellidae.—Central tooth of radula tricuspid, median cusp strong, lateral bicuspid, cusps unequal (Fig. 117, p. 220); shell fusiform or pear-shaped, heavy, canal often long, operculum corneous, claw-shaped, nucleus terminal. Miocene——. Principal genera: Turbinella, Cynodonta, Tudicla (subg., Streptosiphon); Piropsis (Cretaceous), Perissolax (Cretaceous), Strepsidura (Eocene, subg., Whitneya), Melapium, Fulgur (= Busycon, Fig. 150, p. 249, including Sycotypus), Melongena (subg., Pugilina, Myristica); Liostoma (Eocene), Hemifusus (subg., Megalatractus), Ptychatractus, Meyeria.
Fig. 280.—Turbinella pyrum Lam., Ceylon. × ⅔.
Fam. 7. Fasciolariidae.—Eyes at the outer base of the tentacles (radula, Fig. 121, p. 221); shell fusiform, spire long, canal often very long, columella often with a fold at the base; operculum corneous, nucleus terminal. Cretaceous——. Principal genera: Fusus (including Sinistralia, Aptyxis, Troschelia), with subg., Serrifusus (Cretaceous), Clavella (subg. Thersites), Fasciolaria, Latirus (subg. Polygona, Peristernia, Leucozonia, Lagena; Mazzalina (Eocene), Chascax).
Fig. 281.—Latirus (Leucozonia) cingulatus Wood, Panama.
Fam. 8. Mitridae.—Siphon rather long, with anterior appendages, eyes on the side of the tentacles, proboscis very long; radula variable, laterals sometimes lost (Fig. 120, p. 221); shell fusiform, solid, spire more or less pointed, columella with several prominent folds, the posterior the largest, aperture rather[425] narrow, no operculum. Cretaceous——. Principal genera: Mitra (with many sections), subg., Strigatella, Mitreola, Mutyca, Dibaphus; Plochelaea (Tertiary), Thala; Turricula (with several sections), Cylindromitra, and Imbricaria.
Fam. 9. Volutidae.—Foot broad in front, head laterally dilated into lobes, on which are placed the sessile eyes; siphon prominent, with appendages at the base (radula, Fig. 122, p. 221); shell thick, often shining, fusiform, globular or cylindrical, columella projecting anteriorly, with several folds, the anterior of which is the largest, aperture notched, canal not produced, operculum generally absent. Cretaceous——. Principal genera: Cryptochorda (Eocene), Zidona, Provocator, Guivillea, Yetus (= Cymbium), Voluta (with many sections), Volutolithes (chiefly Eocene), Volutolyria, Lyria, Enaeta, Volutomitra.
Fam. 10. Marginellidae.—Foot broad, siphon without appendages, mantle largely reflected over the shell; radula without laterals, central tooth comb-like, cusps rather blunt; shell oval or conoidal, polished, aperture narrow, outer lip thickened, columella with many folds; no operculum. Eocene——. Principal genera: Marginella, with many sections and so-called sub-genera; Persicula, Pachybathron (?), Cystiscus, Microvoluta.
Fig. 282.—Voluta nivosa Lam., West Australia. × ⅔.
Fig. 283.—Oliva porphyria Lam., Panama.
Fam. 11. Harpidae.—Foot large, with a transverse groove, separating off a semi-lunar propodium; mantle partly reflected over the shell; shell ventricose, polished; spire short, strongly longitudinally ribbed, ribs prolonged over the suture, columella callous; no operculum. Eocene——. Single genus, Harpa (subg., Silia).
Fam. 12. Olividae.—Propodium semi-lunar, with a longitudinal groove above, mesopodium reflected laterally over the shell; central tooth of radula tricuspid on a very broad base, lateral simple, hooked; shell sub-cylindrical or fusiform, polished; aperture narrow,[426] operculum present or absent. Cretaceous——. Principal genera: Oliva (Figs. 283, and 98, p. 199), Olivancillaria (including Lintricula and Agaronia), Olivella, Ancilla (subg., Ancillina).
(e) Toxoglossa (p. 218).—Radula with normal formula 1·0·1, teeth large; oesophagus with a large poison gland; animal carnivorous, exclusively marine.

Fig. 284.—Terebra subulata L., Ceylon.
Fig. 285.—Pleurotoma tigrina Lam., E. Indies.
Fam. 1. Terebridae.—Eyes at the end of the tentacles, shell subulate, many whorled, operculum with terminal nucleus. Eocene——. Single genus, Terebra, with several sections.
Fam. 2. Conidae.—Eyes on outer side of tentacles, siphon prominent; shell conical or fusiform, aperture narrow. Cretaceous——. Principal genera: Conus, shell solid, spire short, aperture narrow, straight, internal partitions partly absorbed; Conorbis, Genotia (with several sections, chiefly Tertiary), Pusionella, Columbarium, Clavatula, Surcula, Pleurotoma; Borsonia (Eocene), Drillia (subg., Spirotropis), Bela, Mangilia (including Daphnella, Clathurella, and others), Halia.
Fam. 3. Cancellariidae.—Proboscis short, usually no radula, shell oval, columella strongly plicate; no operculum. Cretaceous——. Single genus, Cancellaria (subg., Merica, Trigonostoma, Admete).
[427]
Visceral loop not twisted (except in Actaeon) in a figure of 8 (Euthyneurous type, p. 203), auricle usually behind the ventricle, ctenidium often replaced by secondary branchiae, pallial cavity, if existing, more or less open, shell present or absent, operculum absent (except in Actaeon), animal hermaphrodite, with separate sexual openings, marine only.—Carboniferous to present time.
The character of their nervous system decisively removes the Opisthobranchiata from the Prosobranchiata, and approximates them to the Pulmonata. Actaeon, however, which is streptoneurous, as well as possessing an operculate shell with prominent spire, forms an interesting link with the Prosobranchiata. At the opposite extreme to Actaeon stand forms like Siphonaria and Gadinia, which are probably close links with the Pulmonata (p. 19). The generative system of the whole group, which is, as in the Basommatophora, of the hermaphrodite type, without mutual fecundation, is another link of connexion with the Pulmonata. The respiratory organs present the most varied forms, sometimes consisting of one ctenidium (never two), sometimes of secondary branchiae, variously placed, while sometimes no special organ exists.
The prolongation of the foot into lateral epipodia or parapodia (possibly to aid in swimming), and the effect of the epipodia upon the shell, according as they involve it completely or partially, are among the most instructive features of the Opisthobranchiata. If the epipodia are developed on the anterior[428] portion of the body, and do not become reflected, they may, as in most Pteropoda Thecosomata, not directly affect the shell. But when, as in the Tectibranchiata, the epipodia are medio-lateral, and tend to envelope the shell, their effect may be traced by a series of forms varying in proportion to the amount of shell-surface covered by the epipodia. The two principal lines along which modification takes place are the gradual reduction of the spiral nature of the shell, and the gradual lessening of its solidity. Both these changes are the direct result of the additional protection afforded to the visceral mass by the reflected epipodia, which renders the existence of a shell less and less necessary. A precisely similar line of change is seen in the Pulmonata, culminating in forms like Arion (p. 174).
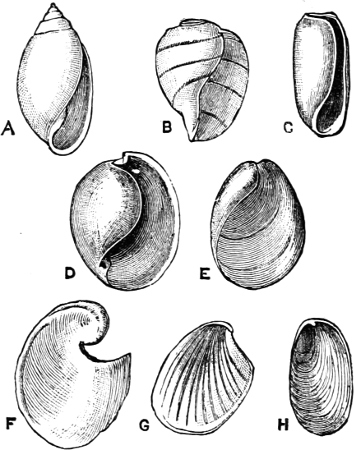
Fig. 286.—Illustrating the transition of form in the shell of Tectibranchiata from the pointed spiral to the almost flattened plate: A, Actaeon; B, Aplustrum; C, Cylichna; D, Atys; E, Philine; F, Dolabella; G, Aplysia; H, Pleurobranchus. (Not drawn to scale.)
Fig. 287.—Illustrating the gradual covering of the shell in the Tectibranchiata by the epipodia and mantle: A, Haminea; B, Scaphander; C, Aplustrum; D, Aplysia; E, Philine; c.d, cephalic disc; ep, ep, epipodia; sh, shell. (Not drawn to scale.)
The habits of life of the Opisthobranchiata are very varied. Some, especially the heavier types, burrow in sand, and are then usually furnished with a broad cephalic disc, as a digging apparatus; some (certain Bulla) flit about in shallow pools on mud flats; others (Phyllirrhoe and the Pteropoda) swim freely in the open sea; others (most Nudibranchiata) crawl slug-like on sea-weeds or corallines, and in colour singularly harmonise with[429] their environment (p. 71 f.); others again (Siphonaria, Gadinia), stick limpet-like to rocks between tide marks. As a rule, they occur only in clean salt water, but Embletonia has been found in the Victoria Docks at Rotherhithe, as well as in parts of the Baltic, where the water has only 7 parts of salt in 1000, while Limapontia occurs in nearly fresh water at Bornholm and Gothland.
Their food varies greatly. As a rule, they are frugivorous, but many cases of carnivorous habit occur. Scaphander has been seen to swallow Dentalium six at a time, and in six hours the shells of all were reduced to tiny fragments. Glaucus devours the soft portions of the pelagic Porpita and Velella; Idalia elegans eats its way into the test of Ascidians, and completely buries itself in the body of its prey.[403]
The Opisthobranchiata may be classified as follows:—
| Opisthobranchiata | 1. Tectibranchiata | Bulloidea |
| Aplysioidea | ||
| Pleurobranchoidea | ||
| Siphonarioidea | ||
| 2. Ascoglossa | ||
| 3. Nudibranchiata | Cladohepatica | |
| Holohepatica | ||
| 4. Pteropoda | Thecosomata | |
| Gymnosomata |
Sub-order I. Tectibranchiata.—Right ctenidium usually present, more or less concealed by the mantle fold, visceral ganglia united by a very long commissure, shell variable in form, more or less enveloped in folds of the mantle and foot, often becoming rudimentary.
Section I. Bulloidea.—Shell more or less spiral, internal or external, epipodia more or less developed, a broad cephalic disc, distinct from the dorsal region, usually no tentacles, eyes sessile.
Fam. 1. Actaeonidae.—Shell spiral, solid, entirely covering the animal; spire generally prominent, operculum corneous, visceral loop streptoneurous, no epipodia, radula multiseriate, teeth numerous, very small. Carboniferous ——. Genera: Actaeon (Fig. 286a); Volvaria (Tertiary), Fortisia (Eocene)[430] Actaeonina (Carboniferous), Cylindrites (Secondary strata), Actaeonella (Cretaceous).
Fam. 2. Tornatinidae.—Shell spiral, cylindrical, entirely covering the animal; spire concealed, cephalic disc with two large tentaculiform appendages behind, no radula. Genera: Tornatina (= Utriculus), Volvula.
Fam. 3. Scaphandridae.—Shell more or less external, covering all or nearly all the animal, spire concealed, cephalic disc simple or notched behind, epipodia well developed, radula with first lateral very large, stomach sometimes with powerful gizzard. Genera: Scaphander (Fig. 287 B); Sabatia (Pliocene), Smaragdinella, Atys (Fig. 286 D), Cylichna (Fig. 286 C), Amphisphyra.
Fam. 4. Bullidae.—Shell external or partly internal, spire quite or nearly hidden, cephalic disc broad, without appendages, epipodia often large; radula usually multiseriate. Genera: Bulla (subg. Haminea), Acera, mantle with long filiform appendage, epipodia touching over the shell; Cylindrobulla, Volvatella.
Fam. 5. Aplustridae.—Shell partly internal, overlaid by the posterior part of the cephalic disc, spire not prominent, epipodia reflected, tentacles auriform. Single genus, Aplustrum (Fig. 286 B; subg. Hydatina).
Fam. 6. Ringiculidae.—Shell small, solid, covering all the animal; spire somewhat prominent, aperture narrow, plicated; peristome thick, sometimes channelled, cephalic disc with a kind of posterior siphon. Genera: Ringicula; Avellana (Cretaceous).
Fam. 7. Gastropteridae.—Shell completely internal, nautiloid, small; epipodia very large, rounded, united behind; cephalic disc simple. Single genus, Gastropteron.
Fam. 8. Philinidae.—Shell completely internal, thin, slightly spiral; epipodia thick, cephalic disc large, thick, simple; stomach usually with powerful gizzard. Genera: Philine (Fig. 287 E), Colpodaspis, Colobocephalus, Chelinodura, Phanerophthalmus, Cryptophthalmus.
Fam. 9. Doridiidae.—Shell completely internal, a mere pellicle with a small spiral nucleus, mantle with two posterior lobes and a caudal filament, epipodia reflected. Single genus, Doridium.
Section II. Aplysioidea.—Shell small, usually not spiral, sometimes absent, no cephalic disc, head prominent, with two pairs of tentacles, epipodia large, more or less reflected.
[431]
Fam. Aplysiidae.—Characters those of the section. Genera: Aplysia (Fig. 287 D), shell arched, flattened, animal large (the “sea hare”); Dolabella, shell subtriangular (Fig. 286 F); Dolabrifer, shell sub-quadrangular, not spiral; Notarchus, shell microscopic, spiral; Phyllaplysia, body very depressed, oval, no shell.
Section III. Pleaurobranchoidea.—Dorsal region protected by a wide notaeum or dorsal covering, or by a shell; no epipodia, ctenidium large, external, between the right under surface of the notaeum or shell and the foot; head short, shell present or absent.
Fam. 1. Pleurobranchidae.—Shell internal or absent, notaeum with spicules, radula multiseriate. Genera: Pleurobranchus (Fig. 286 H), (?) Haliotinella, Pleurobranchaea, (?) Neda.
Fam. 2. Runcinidae.—Branchial lamellae few, under the posterior right notaeum, no shell. Single genus, Runcina.
Fam. 3. Umbrellidae.—Shell external, depressed patelliform, not covering all the animal; foot very thick, ctenidium large, head depressed, small; radula multiseriate, teeth innumerable, very small. Genera: Umbrella (Fig. 5A, p. 10), Tylodina.
Section IV. Siphonarioidea.—Shell patelliform, branchia replaced wholly or in part by a pulmonary sac, pulmonary orifice closed by a small lobe, radula multiseriate, teeth very small.
Fam. Siphonariidae.—Characters those of the section. Genera: Siphonaria (branchia as well as pulmonary sac), Gadinia (no branchia). These genera, hitherto placed among the Pulmonata, have been recently shown (see p. 19) to be modified Opisthobranchiata.
Sub-order II. Ascoglossa.[404]—Branchia, mantle cavity, and shell generally wanting, liver ramified, rami enclosed in external papillae (cerata) or beneath the dorsal surface, kidney not compact, branched; radula with one series of strong teeth (Fig. 288), worn out teeth at the front end not dropping off, but preserved in a special sac (ἀσκός).
According to Bergh, the Ascoglossa form a link between the Tectibranchiata,—especially the Aplysiidae and Bullidae—and[432] the Cladohepatic Nudibranchs, while the Pleurobranchidae form a somewhat similar link between the Holohepatic Nudibranchs and the other Tectibranchiata.
Fam. 1. Oxynoeidae.[405]—Animal long, tentacles auriform, epipodia large, simple, or wing-like, a ctenidium and branchial chamber on right side, shell small, thin, slightly spiral, not covering much of the body. Genera: Oxynoe (= Lophocercus), Lobiger.
Fam. 2. Hermaeidae.—Body depressed, cerata in several rows, no branchiae, no shell. Genera: Hermaea, Phyllobranchus, Stiliger, Alderia.
Fam. 3. Elysiidae.—Body depressed, head rather elevated, tentacles auriform, sides of body dilated into two large wings, which enclose branches of the liver and sometimes fold over the dorsal surface, no branchiae, no shell. Genera: Elysia, Thridachia, Placobranchus.
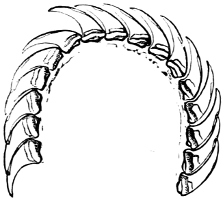
Fig. 288.—Radula of one of Ascoglossa (Elysia viridis Mont. × 40).
Fam. 4. Limapontiidae.—Body slug-like, liver scarcely ramified, no branchiae, shell, or appendages. Genera: Limapontia, Actaeonia, Cenia.
Sub-order III. Nudibranchiata.—Shell absent in the adult, no ctenidium proper, or osphradium, cerata dorsal or dorso-lateral, nervous system concentrated, kidney not compact, ramified, penis retractile, jaws and radula usually present.
Section I. Cladohepatica.—Cerata usually latero-dorsal, elongated, or arborescent, buccal mass strong, jaws present, liver generally ramified, rami generally entering the cerata.
Fam. 1. Aeolidiidae.—Body slug-like, head with tentacles and rhinophores, dorsal area with rows of cerata, which usually contain sting-cells, radula variable. Genera: Aeolis, Cratena, Tergipes, Coryphella, Favorinus, Facelina, Flabellina, Fiona, Glaucus, Janus, Hero, with many sub-genera.
Fam. 2. Tethymelibidae.—Body slug-like, large, cerata very large, no sting-cells, head large, cowl-shaped, no tentacles, rhinophores much foliated, no radula. Genera: Tethys, Melibe. The cerata of Tethys, which are capable of independent movement[433] when severed, have been described as parasitic worms. Tethys feeds on molluscs and Crustacea.
Fam. 3. Lomanotidae.—Body slug-like, dorsum prominent, undulating or lobed, with one row of small cerata, no tentacles, rhinophores much foliated, radula with uncinated dentate laterals. Single genus, Lomanotus.
Fam. 4. Dotonidae.—Body slug-like, small, two rows of cerata, each ceras surrounded by a ring of tubercles, rhinophores simple, radula uniseriate. Single genus, Doto.
Fam. 5. Dendronotidae.—Body slug-like, somewhat compressed, two rows of arborescent cerata, no tentacles, frontal margin with arborescent papillae, rhinophores arborescent, radula multiseriate. Genera: Campaspe, Dendronotus.
Fam. 6. Bornellidae.—Two rows of dorsal papillae, with branchiform appendages at the base, rhinophores foliate, radula multiseriate. Single genus, Bornella.
Fam. 7. Scyllaeidae.—Body oblong, compressed, two large foliated cerata with branchial appendages on the inner side, no tentacles, rhinophores large, radula multiseriate. Single genus, Scyllaea.
Fam. 8. Phyllirrhoidae.—Body much compressed, with bovine head and neck, tail tapering, no tentacles, rhinophores simple, teeth few, no marginals. Single genus, Phyllirrhoe.
Fam. 9. Pleurophyllidiidae.—Body elongate-oval, snout broad, covered by an arched shield with lateral angles prolonged, branchiae consisting of two rows of lamellae placed between the notaeum and the foot, no tentacles, rhinophores short, hidden, radula multiseriate. Single genus, Pleurophyllidia.
Fam. 10. Pleuroleuridae.—Animal resembling Pleurophyllidia, but without the branchial lamellae. Single genus, Pleuroleura.
Fam. 11. Tritoniidae.—Body long, two rows of unequal arborescent cerata, rhinophores with ramose appendages, liver not prolonged into the cerata. Genera: Tritonia, Marionia.
Section 2. Holohepatica.—Cerata medio-dorsal, retractile or not, usually paucifoliate, liver never ramified, usually no jaws.
Fam. 1. Dorididae.—Branchia consisting of a circle or semicircle of pinnate leaves united at the base, surrounding the anus, almost always retractile into a cavity, rhinophores foliate, no suctorial proboscis, radula multiseriate. Genera: Bathydoris,[434] Hexabranchus, Archidoris (Fig. 289), Discodoris, Diaulula, Cadlina, Centrodoris, Platydoris, Chromodoris, Miamira, with many sub-genera.
Fig. 289.—Doris (Archidoris) tuberculata L., Britain: a, anus; br, branchiae surrounding the anus; m, male organ; rh, rh, rhinophores. × ⅔.
Fam. 2. Doriopsidae.—Branchia and rhinophores as in Dorididae, oral aperture pore-shaped, suctorial, no radula. Single genus, Doriopsis.
Fam. 3. Phyllidiidae.—Body oval, depressed, leathery, a ring of branchial lamellae, only interrupted by the head and genital papilla, under the pallial edge, oral aperture pore-shaped, suctorial, no radula. Genera: Phyllidia, Fryeria. Bergh unites this and the preceding family in the group Porostomata, which, with Fam. 1, form the group Dorididae cryptobranchiatae.
Fam. 4. Polyceridae.—Body slug-like, branchiae not retractile, usually surrounding the anus, rhinophores foliate, tentacles simple, radula variable, central tooth generally wanting. Genera: Notodoris, Triopella, Aegires, Triopa, Issa, Triopha, Crimora, Thecacera, Polycerella, Palio, Polycera, Ohola, Trevelyana, Nembrotha, Euplocamus, Plocamopherus, Kalinga.
Fam. 5. Goniodoridae.—Body oval, depressed, branchia multifoliate, usually disposed in shape of a horse-shoe, rhinophores foliate, retractile or not, mouth with a large suctorial proboscis, radula variable. Genera: Akiodoris, Doridunculus, Acanthodoris, Adalaria, Lamellidoris, Calycidoris, Goniodoris, Idalia, Ancula, Drepania.
Fam. 6. Corambidae.—Body otherwise Doris-like, but with two posterior branchiae under the mantle edge, jaws present, no central tooth, about five laterals. Single genus, Corambe (= Hypobranchiaea). Bergh unites this and the two preceding families in the group Dorididae phanerobranchiatae.
Sub-order IV. Pteropoda.—The Pteropoda are pelagic animals in which the lateral portions of the foot are modified into fins, which are innervated by the pedal ganglia. Their systematic position has undergone recent revision. It has been the custom to regard them as an Order of equivalent value to the[435] other four, while some have held them to be a subdivision of Cephalopoda. Modern authorities, chief among whom is Pelseneer, regard the Pteropoda not as a primitive, but as a derived and recent group. They are “Gasteropoda in which the adaptation to pelagic life has so modified their external characters as to give them an apparent symmetry.”
The principal point which relates the Pteropoda to the Gasteropoda is the asymmetry of the visceral organs, intestine, heart, kidney, and genital gland, which results from their development on one side only of the body. Their hermaphroditism and the structure of their nervous system relate them to the Euthyneura rather than to the Streptoneura. Resemblances in the organs of circulation and generation approximate them to the Opisthobranchiata rather than to the Pulmonata, while of the two groups of the former, they tend to closer relationship with the Tectibranchiata than with the Nudibranchiata. The two sections of Pteropoda have been considered of distinct origin, the Thecosomata being derived from the Bulloidea, the Gymnosomata from the Aplysioidea.[406]
Thus the Pteropoda are a group whose true relations are masked by the special conditions of their existence, which have tended towards the development of certain organs, the so-called “wings” and the shell, which give them an apparent symmetry; this symmetry disappears on a closer investigation of the internal organs. They are hermaphrodite; the genital gland has a single efferent duct (except in some Cavolinia), a seminal groove leading to the copulatory organ, which in the Thecosomata is on the right side of the head, in the Gymnosomata on the right side of the foot. The genital system resembles that of the Opisthobranchiata and of the “digonoporous” Pulmonata.
Section 1. Thecosomata.—Shell or cartilaginoid test always present, fins united by an intermediate lobe, ctenidia as a rule absent, replaced by secondary branchiae, no very distinct head or eyes, one pair of tentacles; cerebral ganglia on the sides of and under the oesophagus; radula with three rather large teeth in a row, generally unicuspid, jaw in two pieces, stomach with horny plates, anus generally on the left side.
The Thecosomata feed on Protozoa and the lower Algae;[436] they have no proboscis, and the intestine is flexured. The fins are always closely connected with the head, or what answers to it. About 42 species are known, belonging to 8 genera.
Fam. 1. Limacinidae.—Fins very large, branchial chamber dorsal, anus on right side; shell spiral, sinistral (ultra-dextral, see p. 249), operculate. Genera: Limacina, shell helicoid, deeply umbilicated (L. helicina swarms in Arctic seas and furnishes food for many Cetacea); Peraclis, spire turreted, aperture large, elongated, produced anteriorly, no umbilicus; operculum sinistral, in spite of the shell being ultra-dextral.
Fam. 2. Cavoliniidae.—Fins large, branchial chamber ventral, shell a non-spiral cone, angular or round, very thin, embryonic portion distinct, or formed of two separate plates.
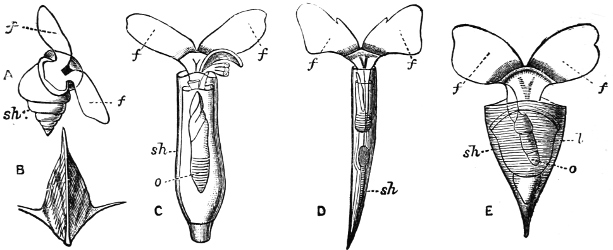
Fig. 290.—Illustrations of Pteropoda Thecosomata: A, Limacina australis Eyd.; B, Cleodora cuspidata Bosc. (shell only); C, Cuvierina columnella Rang; D, Creseis virgula Rang; E, Clio balantium Rang; f, f, fins; l, liver; o, ovary; sh, shell. (After Souleyet.)
In Cavolinia (= Hyalaea, Fig. 5 B, p. 10) the shell consists of two plates, the ventral being convex, with one to three sharp spines at the posterior end, the dorsal flatter, without spines. The aperture is broad, contracted dorso-ventrally. Two long pointed prolongations of the mantle project from the lateral slits of the shell, and probably serve to balance the bulky body when swimming. Fins trilobed at the margin. Cleodora has only rudimentary lateral prolongations, fins bilobed, shell triangular, angles greatly produced, aperture very wide, dorsal side keeled. In Cuvierina the shell is straight, sub-cylindrical, with a median partition, slightly expanding towards the apex, which is truncated in the adult. The principal sub-genera of Clio are Creseis, which has an elongated sub-cylindrical shell, sometimes slightly curved,[437] smooth or grooved; and Clio proper, in which the shell is long, angular, with a dorsal rib, apex (= embryonic shell) rounded, constricted. Styliola and Hyalocylix also belong to this group.
Fam. 3. Cymbuliidae.—Test (which is not homologous with the shell of other Thecosomata) slipper-shaped, cartilaginoid, simply a thickening of the mantle; embryo with a calcareous, spiral, operculate shell. Genera: Cymbulia, Cymbuliopsis, Gleba.
Three other families, Hyalithidae, Pterothecidae, and Conulariidae, from Palaeozoic strata, are generally added to the Thecosomata. All are fossil only, and it is doubtful whether they are really Molluscan. Pelseneer holds that no true fossil Pteropoda occur until the lower Tertiaries.
Section 2. Gymnosomata.—Mantle and shell absent in the adult, fins not connected by a lobe, no branchial chamber, head well developed, with two pairs of tentacles, eyes on the posterior pair; cerebral ganglia above the oesophagus; buccal cavity provided with a pair of protrusible “hook-sacs,” radula generally with 4 to 12 hooked laterals, central tooth triangular, jaw in one piece, composed of horny plates, no horny plates in stomach, anus on the right side.
The Gymnosomata are carnivorous, feeding on Thecosomata and other pelagic animals, being provided for this purpose with a formidable buccal armature of hook-sacs and suckers. The intestine, as usual in carnivorous groups, passes straight from the stomach to the anus; the fins are not attached to the head, but to the anterior part of the body. The larva has a straight shell, which disappears in the adult. About 21 species are known, belonging to 7 genera.
Fam. 1. Pneumodermatidae.—Animal fusiform, fins rather small, head prominent, anterior part of buccal cavity protrusible, with suckers on the ventral side, hook-sacs well marked; branchia on right side, skin soft, pigmented. Genera: Dexiobranchaea, no posterior gill, hook-sacs short; Spongiobranchaea, posterior gill circular; Pneumoderma, gill tetraradiate, hook-sacs long.
Fam. 2. Clionopsidae.—Body barrel-shaped, proboscis three times the length of the body, no buccal appendages; hook-sacs short, no lateral gill, posterior gill tetraradiate, skin not pigmented. Clionopsis is the single genus.
Fam. 3. Notobranchaeidae.—Body ovate, buccal appendages[438] conical, no lateral gill, posterior gill with three radiating crests, skin pigmented. Notobranchaea is the single genus.
Fam. 4. Clionidae.—Body long, angulated behind, proboscis short, mouth with two or three pairs of appendages, no jaw, no gills.
Clione limacina is so abundant in Arctic seas as at times to colour the surface for miles. Each of the cephalic appendages has about 60,000 minute pedicellated suckers.
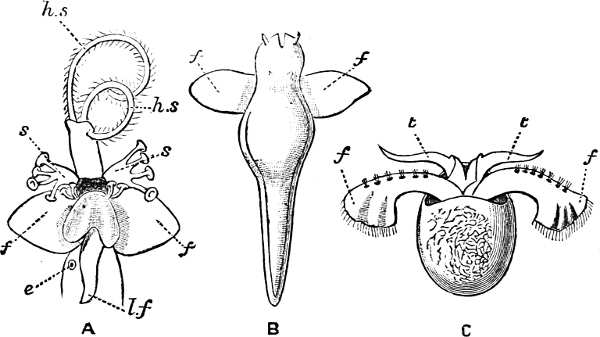
Fig. 291.—A, Anterior portion of Pneumoderma; B, Clione limacina Phipps; C, Halopsyche Gaudichaudi Soul.; f, f, fins; h.s, h.s, hook-sacs; l.f, lobe of the foot; s, s, suckers; o, posterior genital orifice; t, t, tentacles. (After Souleyet.)
Fam. 5. Halopsychidae.—Body ovate, thick, rounded behind, no gill or proboscis, fins long, narrow, broadened at the ends, epidermis sub-cartilaginoid.
Halopsyche (= Eurybia) has the power of withdrawing its head completely into a sort of pocket, which is closed by an anterior fold of the mantle. There are two long non-retractile buccal appendages.
Gasteropoda with two pairs of tentacles, visceral loop euthyneurous, ganglia concentrated round the oesophagus; breathing air by a pallial cavity formed by the union of the front edge of the mantle with the cervical region, sexes united, shell present or absent, no operculum[407] (except in Amphibola).
Sub-order I. Basommatophora.—Eyes generally at the base of the tentacles, which are not retractile, male and female genital orifices separate, radula (p. 235) multiseriate, shell always present, external. Fresh water or quasi-marine.
[439]
Fam. 1. Auriculidae.—Breathing organ a pulmonary sac or true lung; shell spiral, conoidal, internal partitions usually absorbed, aperture more or less strongly toothed. Jurassic——. Genera: Auricula, Carychium, Scarabus, Alexia, Tralia, Plecotrema, Cassidula, Melampus, Leuconia, Pedipes (Fig. 292).
Fig. 292.—Examples of the Auriculidae: A, Auricula Judae Lam., Borneo; B, Scarabus Lessoni Blainv., E. Indies; C, Cassidula mustelina Desh., N. Zealand; D, Melampus castaneus Mühlf., S. Pacific; E, Pedipes quadridens Pfr., Jamaica.
Fam. 2. Otinidae.—Shell auriform, spire very short. Genera: Otina, Camptonyx.—Recent only.
Fam. 3. Amphibolidae.—A pulmonary sac on right side of neck, eyes almost pedunculate, shell turbinate, rudely sculptured, operculate.—Recent. Genus, Amphibola (Fig. 293); subg. Ampullarina.
Fam. 4. Limnaeidae.—Pulmonary sac protected by an external lobe; shell variable, fragile. Jurassic——. (i.) Ancylinae, shell more or less limpet-shaped. Genera: Ancylus, Gundlachia, Latia. (ii.) Limnaeinae, shell spiral. Genera: Limnaea, Amphipeplea, Erinna, Lantzia, Pompholyx, Choanomphalus (with Carinifex). (iii.) Planorbinae, shell sinistral, spire flattened or elevated. Genera: Planorbis, Isidora (= Bulinus).
Fam. 5. Physidae.—Mantle more or less reflected over the shell (radula, Fig. 141C, p. 235); shell sinistral, lustrous. Jurassic——. Genera: Physa, Aplecta.
Fig. 293.—Amphibola avellana Chem.
Fam. 6. Chilinidae.—Lobe of pulmonary sac large, tentacles broad; shell ventricose, rather solid; columella plicate. Miocene——. Single genus, Chilina.
Sub-order II. Stylommatophora.—Two pairs of retractile tentacles (except in Janella), eyes at the tip of the upper pair, male and female orifices united (except in Vaginulidae and Onchidiidae), no distinct osphradium.
Fam. 1. Testacellidae.—Animal carnivorous, slug-like or spirally coiled, no jaw (whence the name Agnatha, often given[440] to this group), radula with usually few, large, sickle-shaped teeth (p. 232), shell variable, rarely absent, usually external. Cretaceous——. Principal genera: Chlamydephorus (shell a simple plate, internal), Apera, Testacella (slug-like, shell terminal), Strebelia, Streptostyla, Glandina, Salasiella, Petenia, Pseudosubulina, Streptostele, Tomostele, Streptaxis (Fig. 203), Gibbus, Ennea, Daudebardia (Fig. 193), Schizoglossa, Guesteria, Aerope, Paryphanta, Rhytida (subg. Diplomphalus, Elaea and Rhenea).
Fig. 294.—A, Ennea (Gibbulina) palanga Fér; A´ young of same; B, Gibbus lyonetianus Pall.
Fam. 2. Selenitidae.—Shell internal, external, or absent; jaw present, radula Testacellidan, central tooth present. Tertiary——. Genera: Selenites, Plutonia, Trigonochlamys, Pseudomilax (?), Rathouisia (?).
Fam. 3. Limacidae.—Shell present or absent, internal or external, spiral or not, tail often with a mucus pore, jaw (Fig. 107 A, p. 211) with projecting rostrum on cutting edge, radula with central tooth tricuspid, laterals bi- or uni-cuspid, marginals aculeate. Eocene——. Genera: Otoconcha, Urocyclus, Mariaella (subg. Tennentia), Parmarion, Helicarion, Cystopelta, Aspidelus, Estria, Vitrinopsis (subg. Vitrinoidea, Parmella), Damayantia, Nanina (= Ariophanta, including Pachystyla, Rhysota, Hemiplecta, Trochonanina, Euplecta, Orpiella, Xesta, Macrochlamys, Microcystis, Sitala, Kaliella, Durgella, Austenia, Girasia, Parmacochlea, Africarion, Sesara, Macroceras, and others), Vitriniconus, Parmacella, Limax (subg. Amalia and many sections), Vitrina (subg. Vitrinozonites, Velifera), Zonites (subg. Stenopus, Moreletia, Mesomphix, Hyalinia, Gastrodonta, Pristiloma, Poecilozonites, Thyrophorella).
Fam. 4. Philomycidae.—Shell absent, jaw limacidan, radula helicidan, shield covering all the body. Single genus, Philomycus (= Tebennophorus), with subg. Pallifera.
Fam. 5. Helicidae.—Shell present or absent, internal or external; jaw of various types, radula with central tooth tricuspid, equal in size to the first laterals, laterals bi- or tricuspid, marginals smaller, cusped. Eocene——. Principal genera: Oopelta (no shell), Arion (shell absent or formed of calcareous granules),[441] Ariolimax, Geomalacus, Anadenus (subg. Prophysaon), Hemphillia, Cryptostracon, Binneya, Helix (see below), Cochlostyla, Bulimus (subg. Borus, Orphnus, Dryptus, Strophochilus, Pachyotus, and possibly Caryodes, Leucotaenia, Liparus, Livinhacea, Pachnodus, Rachis, Atopocochlis, Cerastus, Clavator belong here, or with Buliminus), Berendtia, Rhodea. Pilsbry proposes[408] to group Helix as follows:
A. Eggs or young very large at birth:
(1) Macroön, incl. Acavus, Pyrochilus (= Phania), Stylodonta, Helicophanta.
B. Eggs or young smaller or minute at birth:
(2) Belogona.—Female genital system with dart sac and mucus gland. Helix [restricted] (with sections Arionta, Campylaea, Chilotrema, Pomatia, Macularia, Tachea, Iberus, Leptaxis, Eulota, Fruticicola, Xerophila; Dorcasia, Acusta, Plectotropis, Aegista, Cathaica, Satsuma, Euhadra; Lysinoe), Gonostoma, Leucochroa, Allognathus, Cochlostyla, Polymita, Hemitrochus (with sections Plagioptycha, Dialeuca, Coryda, Jeanerettia), Glyptostoma, Acanthinula, Vallonia.
(3) Teleophalla.—Female system without accessories, male with flagellum and appendix on penis; no epiphallus. Sagda, Cysticopsis.
(4) Epiphallophora.—Female system without accessories, male with epiphallus on penis; no appendix. Caracolus (with sections Lucerna, Dentellaria, Isomeria, Labyrinthus, Eurycratera, Parthena, Polydontes, Theliodomus, Cepolis), Camaena (incl. Phoenicobius), Obba, Chloritis (incl. Hadra), Papuina, Planispira (subg. Cristigibba).
Fig. 295.—Example of the Macroön group of Helix. Helicophanta Souverbiana Fisch., Madagascar, showing large embryonic shell. × ⅔.
(5) Haplogona.—All accessory organs absent, jaw soldered into one piece. Polygyra (incl. Daedalochila, Triodopsis, Mesodon, Stenotrema), Endodonta (incl. Libera, Charopa, Gerontia, Therasia, and others), Patula, Trochomorpha, Anoglypta.
(6) Polyplacognatha.—All accessory organs absent, jaw composed of 16–24 separate plates. Punctum, Laoma.
[442]

Fig. 296.—Odontostomus pantagruelinus Moric., S. Brazil. × ½.
Genera of doubtful position: Strobilops, Ampelita, Pedinogyra, Polygyratia, Macrocyclis, Solaropsis.
Fam. 6. Orthalicidae.—Radula, p. 233. Shell external, large, bulimoid. Single genus, Orthalicus; subg. Liguus, Porphyrobaphe, Corona.
Fam. 7. Bulimulidae.—Radula, p. 233; jaw, p. 211; shell usually external. Genera: Bulimulus (incl. Plecochilus, Goniostomus, Drymaeus, Liostracus, Otostomus, Navicula, Scutalus, Peronaeus, Eurytus, Eudioptus, Plectostylus, Mesembrinus, Mormus, etc.; Thaumastus, Nesiotis), Placostylus (incl. Charis), Amphidromus, Partula, Calycia (?), Peltella (animal limaciform, shell internal), Pellicula, Amphibulimus (incl. Simpulopsis).
Fam. 8. Cylindrellidae.—Radula, p. 233; shell many whorled, long turriculate, last whorl often detached, apex often truncated (Fig. 169, p. 260). Eocene——. Genera: Cylindrella (with sections Callonia, Thaumasia), Leia, Macroceramus, Pineria.
Fam. 9. Pupidae.—Radula, p. 233; shell external, spire usually long, aperture often narrowed, more or less toothed, often with internal lamellae. Carboniferous——. Genera: Anostoma (Fig. 154, p. 248), Hypselostoma (Fig. 202A, p. 302); Anastomopsis (Cretaceous), Lychnus (Cretaceous), Boysia, Odontostomus (incl. Tomigerus), Buliminus (incl. Petraeus, Napaeus, Zebrina, Mastus, Chondrula, Ena, and perhaps Rachis, Pachnodus, Hapalus, and others), Pupa (incl. Torquilla, Pupilla, Sphyradium, Leucochila, etc.), Zospeum, Vertigo, Megaspira, Strophia, Holospira, Eucalodium (incl. Coelocentrum), Coeliaxis, Perrieria, Balea; Rillya (Eocene), Clausilia (with many sub-genera), Rhodina (?).
Fig. 297.—A, Clausilia crassicosta Ben., Sicily; B, Clausilia macarana Zieg., Dalmatia; B´, clausilium of same.
Fam. 10. Stenogyridae.—Radula, p. 234; shell long, spiral, shining, more or less translucid, apex blunt, sometimes decollated. Eocene——. Genera: Stenogyra (subg. Rumina, Obeliscus, Opeas, Melaniella, Spiraxis, Leptinaria, Nothus, Subulina, Glessula), Ferussacia (subg. Cionella, Azeca), Caecilianella (subg. Geostilbia). Achatina (shell large, ventricose,[443] columella strongly truncate), with the sub-genera Perideris, Limicolaria, Columna, Pseudachatina, Homorus, probably belongs to a distinct family.
Fam. 11. Achatinellidae.—Radula, p. 234; shell small, bulimoid, indifferently dextral or sinistral. Genera: Achatinella (subg. Auriculella, Amastra, Carelia), Tornatellina.
Fam. 12. Succineidae.—Radula, p. 234; lower pair of tentacles wanting or small; shell internal or external, thin, spiral or not, last whorl large. Eocene——. Genera: Succinea, Homalonyx, Hyalimax, (?) Lithotis, (?) Catinella.
Fam. 13. Janellidae.—Radula, p. 234; animal slug-like, no lower tentacles, shell an internal plate. Single genus, Janella (= Athoracophorus), with subg. Aneitea.
Fam. 14. Vaginulidae.—Radula, p. 234; animal slug-like, covered with a coriaceous mantle, lower tentacles bifid, genital orifices widely separated, male behind the lower right tentacle, female on inferior median part of right side, anus and pulmonary orifice nearly terminal; shell absent. Single genus, Vaginula (= Veronicella).
Fig. 298.—Achatina zebra Lam., S. Africa. × ½.
Fam. 15. Onchidiidae.—Body oval, mantle thick, often warty, sometimes set with “eyes” (p. 187), two tentacles, genital orifices widely separate, anus and pulmonary orifice as in Vaginula; no shell. Genera: Peronia, Onchidium, Onchidiella. The family appears to be an instance of Pulmonata reverting to marine habits of life.
[444]
Head rudimentary, mantle edges ventrally concrescent, forming a tube opening before and behind, and covered with a shell of the same shape; sexes separate.
Fig. 299.—Anatomy of Dentalium: a, anterior aperture of mantle; f, foot; g, genital gland; k, kidney; l, liver. (After Lacaze-Duthiers.)
The Scaphopoda form a small but very distinct class, whose organisation is decidedly of a low type. The body is usually slightly curved, the concave side being the dorsal; muscles near the posterior end attach the body to the shell. The foot, which can be protruded from the anterior or wider aperture, is rather long, pointed, and has sometimes two lateral lobes (Dentalium), sometimes a terminal retractile disc (Siphonodentalium), sometimes a retractile disc with a central tentacle (Pulsellum). The cephalic region, as in Pelecypoda, is covered by the mantle. The mouth is situated on a kind of projection of the pharynx; the buccal mass, containing the radula (p. 236), is at the base of the foot, and the intestine branches forward from the front part of the stomach. The liver (Fig. 299) is paired, and consists of a number of symmetrical, radiating coeca. There are no eyes, but on each side of the mouth are small bunches of exsertile filaments (captacula), which appear to act as[445] tactile organs for the seizing of food. There is no special respiring apparatus, heart or arterial system, breathing being conducted by the walls of the mantle. The nervous system has already been described (p. 205).
Two kidneys open on either side of the anus. The genital gland is large, occupying nearly all the posterior part of the body, the sexual products being emitted through the right kidney. The veliger has already been figured (p. 131, Fig. 44). The embryonic shell is formed of two calcareous laminae, which subsequently unite to form the tube.
With regard to their general relationships, the Scaphopoda resemble the Gasteropoda in their univalve shell, and in the possession of a radula; while the pointed foot, the non-lobed velum in the veliger, the generative system, the bilateral symmetry of the organs generally, and the absence of any definite head, eyes, or tentacles, are points which approximate them to the Pelecypoda.
The Scaphopoda are known from Devonian strata to the present time. They are found at a depth of a few fathoms to very deep water. The only three genera are Dentalium, Siphonodentalium (subg. Cadulus), and Pulsellum, which differ in the structure of the foot, as described above.
Cephalic region rudimentary, mantle consisting of two symmetrical right and left lobes, covering the body and secreting a bivalve shell hinged at the dorsal margin; no radula, sexes usually separate. Reference has already been made to the reproductive system (p. 145), breathing organs (p. 164 f.), mantle (p. 172), nervous system (p. 205), digestive system (p. 237 f.), and nomenclature of the various parts of the shell (p. 269 f.).
The shape of the shell, in many Pelecypoda, involving as it does the position, size, and number of the adductor muscles, is probably due to mechanical causes, depending on the habits and manner of life of the individual genus. Thus in a typical dimyarian or two-muscled bivalve, e.g. Mya (Fig. 300, A), the adductor muscles lie well towards each end of the long axis of the shell, with the hinge about midway between them. In this position they are best placed for effectually closing the valves,[446] and since they are nearly equidistant from the axis of motion, i.e. from the hinge, they do an equal amount of work, and are about equal in size. But in a form like Modiola, where the growth of the shell is irregular in relation to the hinge-line, the anterior muscle is brought nearer and nearer to the umbones, where its power to do work, and therefore its size, becomes less and less. But the work to be done remains the same, and the posterior muscle has to do it nearly all; hence it moves farther and farther away from the hinge-line, and at the same time gains in size. In shells like Ostrea, Pecten, and Vulsella, the anterior muscle, having drawn into line with the hinge and the posterior muscle, becomes atrophied, while the posterior muscle, having double work to do, has doubled its size.[409]
Fig. 300.—Illustrating changes in the position and size of the adductor muscles according to the shape of the shell: A, Mya; B, Modiola; C, Vulsella. The upper dotted line shows the hinge-line, the lower connects the two muscles.
The development of the foot, again, largely depends upon habits of life. It is well developed in burrowing forms, while in sessile genera (Ostrea, Chama, Spondylus) it becomes unnecessary and aborts. Even in Pecten, which does not become sessile, but has ceased to use the foot as an organ of progression, a similar result follows. Forms which burrow deeply often “gape” widely, sometimes at one end only, sometimes at both. Venus, Donax, Tellina, Mactra, which are shallow burrowers, do not gape; Solen, Lutraria, and to a less degree Mya, burrow deeply and gape widely. In order to burrow deeply the foot must be highly developed, and the larger it becomes, the more will it tend to keep the valves apart at the place where it is habitually protruded. Burrowing species always remain in communication with the surface by means of their siphons, the constant extension of which tends to keep the valves apart at the end opposite to the foot. Burrowing[447] species, again, tend to burrow in such a way as to descend most easily, and not be impeded by their own shells; in other words, they act as a wedge, and descend with their narrowest part foremost. But the burrowing organ, the foot, has to follow suit, and gradually draws round to the narrowest part of the shell, so that the habitual deep burrower, such as Lutraria, lies with its long axis exactly at right angles to the surface, its siphons protruding from, and keeping open, the uppermost or posterior margin of the shell, and the foot producing the same effect upon the lower or anterior margin. The deeper the burrower, the more elongated does the shell become, until, through forms like Pholas and Saxicava, we arrive at Solen, the most highly specialised burrower of all, in which the breadth of the shell is equal throughout, and no obstructive curve exists to impede its rapid ascent or descent.
The Pelecypoda have been classified in various ways; by the completeness or sinuation of the pallial line, depending on the absence or presence of siphons, by the number of adductor muscles, by the character of the hinge-teeth, and by the number of the branchiae. For various reasons, none of these methods have proved entirely satisfactory. That adopted here was suggested by Pelseneer, and depends upon the character of the branchiae themselves, as suggesting successive stages of development (p. 166 f.).
Branchial filaments not reflected, the two rows inclined at a right angle (more or less), ventral surface of foot more or less flattened, byssogenous apparatus little developed, a single anterior aorta, kidneys distinct, sexes separate, each genital gland opening into the corresponding kidney.
Fam. 1. Nuculidae.—Labial palps very large, rows of branchial filaments at right angles to one another, mantle edges open, siphons contracted, foot disc-shaped, elongated; shell equivalve, oval, or produced, interior generally nacreous, hinge with numerous saw-like teeth. Silurian ——. Principal genera: Nucula (heart dorsal to the rectum); Palaeoneilo (Devonian), (?) Sarepta, Leda, Yoldia, Malletia; Tyndaria (Upper Tertiary), Lyrodesma (Silurian), Actinodonta (Silurian), Babinka (Silurian).
Fam. 2. Solenomyidae.—Labial palps united, one row of[448] branchial filaments pointing dorsally, the other ventrally; mantle edges in great part united postero-ventrally, a single siphonal orifice with two very long tentacles, foot proboscidiform, with a round denticulate disc at the end; shell equivalve, resembling a Solen, with a strong corneous periostracum; no hinge-teeth, ligament internal. Single genus, Solenomya. (?) Cretaceous ——].
Rows of branchial filaments parallel, pointing ventrally, reflected, and provided with interfilamentary ciliated junctions, foot usually with a well-developed byssogenous apparatus.
Sub-order I. Anomiacea.—Heart dorsal to the rectum, a single aorta, foot small, anterior adductor very small; shell ostreiform, no hinge-teeth, fixed by a calcified byssus traversing the right valve (Fig. 173, p. 262).
Fam. Anomiidae.—Jurassic ——. Genera: Anomia, Placunanomia; Carolia (Eocene), Placuna; Hypotrema (Jurassic), Placunopsis (Oolite).
Sub-order II. Arcacea.—Mantle edge open, both adductors well developed, heart with two aortae, branchiae free, without interlamellar junctions, no siphons; renal and generative apertures distinct.
Fam. 1. Arcadae.—Mantle edge with composite eyes; shell round or trapezoidal, solid, often with stout bushy periostracum; ligament often external, on a special area; hinge with numerous lamelliform teeth. Ordovician ——. Principal genera: Arca (incl. Barbatia, Scaphula, and Cucullaea), heart dorsal to rectum; Pectunculus, Glomus, Limopsis; Trinacria and Nuculina (Tertiary).
Fam. 2. Trigoniidae.—Foot large, hatchet-shaped, with ventral disc; no byssus, mantle edge with ocelli; shell sub-trigonal, hinge-teeth few, strong; interior violet-nacreous. Devonian ——. Genera: Trigonia; Myophoria and Schizodus (Trias), Cyrtonotus (Devonian).
Fig. 301.—Trigonia pectinata Lam., Sydney, N.S.W.
Sub-order III. Mytilacea.—Mantle edges fused at one point, anal orifice distinct, anterior terminal adductor small, one aorta,[449] branchiae with interfoliary junctions, genital glands penetrating the side of the mantle and opening by the side of the kidneys.
Fam. Mytilidae.—Byssus well developed, shell more or less equivalve, oval, broad; hinge-teeth evanescent. Devonian——. Principal genera: Mytilus?, Myalina, Septifer, Modiola, Lithodomus, Crenella, Dacrydium, Myrina, Idas, Modiolaria, Modiolarca.
Mantle edges entirely open, foot little developed, anterior adductor usually aborted, branchial filaments reflected, with interlamellar junctions, which are sometimes vascular; genital glands opening into the kidneys or close to the apertures of the kidneys.
Fam. 1. Aviculidae.—Foot long, tongue-shaped, byssogenous apparatus well developed, branchiae concrescent with the mantle, adductor muscle sub-central, at times a small anterior adductor, siphons absent; shell usually inequivalve, dorsal margin straight, often very long, winged, lateral teeth much prolonged; structure of shell cellular, inside prismatic, outside nacreous. Palaeozoic ——. Principal genera: Avicula, including Meleagrina, Malleus; Vulsella (no wings or hinge-teeth); Perna, including Crenatula, Inoceramus (ligaments in a number of fossettes); Aucella and Monotis (Palaeozoic and Secondary); Pterinaea and Ambonychia (Palaeozoic); Pinna; Aviculopinna (Carboniferous).
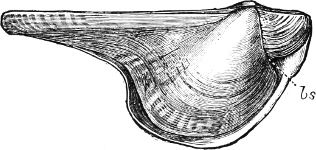
Fig. 302.—Avicula heteroptera Lam., Australia, showing the inequivalve shell and byssal sinus (bs).
Fam. 2. Prasinidae.—Shell very small, umbones anterior, incurved, anterior side depressed, hinge-teeth replaced by dentiform projections of the lunule fitting into corresponding grooves. Recent. Single genus, Prasina.
Fam. 3. Ostreidae.—Heart generally ventral to the rectum, branchiae concrescent with the mantle, no byssus; shell inequivalve, fixed by the left valve, form irregular. Jurassic ——. Genera: Ostrea; Heligmus (Oolite), Naiadina (Cretaceous), Pernostrea (Jurassic).
Fam. 4. Pectinidae.—Byssus usually absent, mantle edge[450] open, duplicated, folded back, with pallial ocelli; branchiae not concrescent with the mantle; shell with unequal “ears” at the umbo, hinge-teeth lamelliform, often obscure. Silurian——. Principal genera: Pedum, Chlamys, Hinnites, Hemipecten, Amussium, Pecten; Aviculopecten (Palaeozoic), Crenipecten.
Fam. 5. Limidae.—Mantle edge as in Pecten, tentaculate; shell sub-equivalve, eared, fixed by a byssus or free. Carboniferous——. Genera: Lima (Fig. 85, p. 179), Limea.
Fam. 6. Spondylidae.—Foot with a peduncular appendage, no byssus, numerous pallial ocelli; shell fixed by right valve, surface often very spinose, two cardinal teeth in each valve. Jurassic——. Genera: Plicatula, Spondylus; Terquemia (Lias).
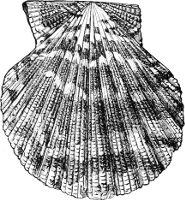
Fig. 303.—Pecten pallium L., East Indies.
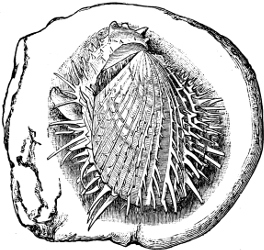
Fig. 304.—Spondylus petroselinum Sowb., Mauritius; on a coral.
Fam. 7. Dimyidae.—Shell ostreiform, fixed, hinge with or without symmetrical teeth, two muscular impressions. Single genus, Dimya (Tertiary).
[451]
Mantle edges united at one or more points, branchiae with interfilamentary junctions which are always vascular, genital glands not opening into the kidneys, usually two adductor muscles.
Sub-order I. Submytilacea.—Mantle edges more or less open, anal orifice distinct, usually no siphons, pallial line usually simple, cardinal and lateral teeth well marked.
Fam. 1. Carditidae.—Foot with a byssus or groove, branchiae large, unequal; shell equivalve, solid, radiately grooved, one or two oblique cardinal teeth, one or two laterals. Silurian ——. Principal genera: Venericardia, Cardita, Carditella, Carditopsis, Milneria; Pleurophorus (Palaeozoic), Anodontopsis (Silurian).
Fam. 2. Astartidae.—A short anal siphon, labial palps large; shell triangular, thick, ligament external, hinge with two or three cardinals in each valve, laterals obscure.? Devonian ——. Principal genera: Astarte; Pachytypus (Jurassic), Plesiastarte (Eocene), Parastarte, Woodia, Opis (Secondary strata), Prosocoelus (Devonian).
Fam. 3. Crassatellidae.—Mantle with anal orifice or open; shell equivalve, thick, subtriangular, ligament in an internal fossette, hinge with two cardinals, laterals produced. Cretaceous——. Principal genus, Crassatella.
Fam. 4. Cardiniidae.—Shell equivalve, oval or triangular, ligament external, cardinal teeth small, laterals fairly strong. Devonian——Oolite. Principal genera: Cardinia, Anthracosia, Carbonicola, Anoplophora.
Fam. 5. Cyprinidae.—Anal and branchial orifices complete, papillose, foot thick; shell variable, equivalve, thick, umbones often spiral, hinge-teeth very variable, ligament external. Jurassic——. Principal genera: Cyprina; Pygocardia (Crag), Veniella (Cretaceous), Venilicardia (Secondary strata), Anisocardia (Jurassic), Isocardia, Libitina, Coralliophaga; Basterotia (Eocene). The families Pachydomidae (Palaeozoic) and Megalodontidae (Palaeozoic—Secondary) are probably related to the Cyprinidae.
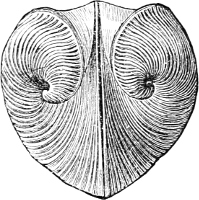
Fig. 305.—Isocardia vulgaris Reeve, China.
Fam. 6. Aetheriidae.—Anal orifice complete, foot absent,[452] labial palps large; shell irregular, free or fixed, no hinge-teeth. Fluviatile, recent only. Genera: Aetheria, Mülleria, Bartlettia.
Fam. 7. Unionidae.—Foot large and thick, no byssus, anal siphon short, branchial orifice complete or not, siphon present or absent, embryo of certain groups passing through a glochidium stage (p. 146); shell equivalve, sometimes very thick, nacreous within, hinge variable. Fluviatile. Jurassic——. Principal genera: Unio (subg. Arconaia), Monocondylaea, Pseudodon, Anodonta, Solenaia, Mycetopus, Mutela, Spatha, Hyria, Castalia, Leila.
Fam. 8. Dreissensiidae.—Both siphons prominent, foot tongue-shaped, byssiferous; shell mytiliform, with small internal septum. Genera: Dreissensia; Dreissensiomya (Tertiary). The common Dreissensia polymorpha Pall. was distributed over large parts of Europe in later Tertiary times. From unknown causes it died out, and has during the past two hundred years been regaining its position, migrating N. and W. from its original habitat, the Caspian, by the Volga and its Oka confluent.
Fam. 9. Modiolopsidae.—Shell mytiliform, ligament exterior, hinge-teeth small, rather numerous. Palaeozoic——. Principal genera: Modiolopsis, Cyrtodonta, Mytilops, Ptychodesma.
Fam. 10. Lucinidae.—Anal orifice sometimes with a siphon, branchial orifice complete or not, sometimes a single branchia; foot very long, vermiform, no byssus, anterior adductor long; shell rounded, equivalve, blanched, hinge with two cardinals and two laterals in each valve, sometimes toothless, ligament more or less internal. Silurian——. Principal genera: Lucina, Corbis, Axinus, Diplodonta, Montacuta.
Fam. 11. Ungulinidae.—Anal orifice complete, foot vermiform, no byssus, two branchiae; shell equivalve, subcircular, hinge-teeth variable, no laterals, adductor impressions long, continuing the pallial line. Tertiary——. Single genus, Ungulina.
Fam. 12. Unicardiidae.—Shell equivalve, round or oval, cardinal shelf large, a single cardinal in each valve, ligament external. Carboniferous—Cretaceous. Genera: Unicardium, Scaldia, Pseudedmondia.
Fam. 13. Kellyellidae.—Anal siphon prolonged, no marked branchial orifice; shell very small, oval or round, anterior lateral very strong, under the cardinal. Eocene——. Genera: Kellyella; Allopagus and Lutetia (Tertiary), Turtonia.
Fam. 14. Erycinidae.—Mantle edges with three apertures,[453] branchial orifice on the buccal margin, foot long, broadened, with a byssus, animal usually viviparous. Tertiary——. Genera: Erycina, Kellia, Pythina, Lasaea, Lepton.
Fam. 15. Galeommidae.—Mantle edges more or less reflected over the shell, apertures and foot as in Erycinidae; shell thin, equilateral, hinge with few teeth or none. Tertiary——. Genera: Galeomma, Scintilla, Sportella, Chlamydoconcha, Hindsiella, Ephippodonta (Fig. 32, p. 81).
Fam. 16. Cyrenidae.—Siphons short, foot large, no byssus; shell equivalve, subtriangular, with periostracum, hinge with two or three cardinals, laterals present; animal hermaphrodite, viviparous. Fresh or brackish water. Jurassic——. Genera: Cyrena, Corbicula (subg. Batissa, Velorita), Sphaerium (= Cyclas), Pisidium, Galatea, Fischeria.
The families Cyrenellidae (single genus, Cyrenella) and Rangiidae (single genus, Rangia) are probably to be placed here.
Sub-order II. Tellinacea.—Siphons long, separate, foot and labial palps very large, pallial sinus deep, two adductor muscles.
Fam. 1. Tellinidae.—External branchial fold directed dorsally, foot with byssogenous slit, but no byssus, branchiae small; shell compressed, equivalve, ligament external, at least two cardinals in each valve, laterals variable. Cretaceous——. Principal genera: Tellina (with many sections), Gastrana.
Fam. 2. Scrobiculariidae.—Animal as in Tellina; shell orbiculate or long oval, equivalve, hinge-teeth weak, ligament in an internal cavity. Tertiary——. Principal genera: Scrobicularia, Syndosmya, Theora, Cumingia, Semele.
Fam. 3. Donacidae.—External branchial fold directed ventrally; shell equivalve, subtriangular, solid, smooth, two or three cardinals in each valve, laterals variable, ligament external. Jurassic——. Genera: Donax, Iphigenia, Isodonta.
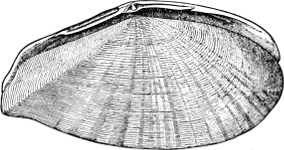
Fig. 306.—Tellina rastellum Hanl., East Indies.>
Fam. 4. Tancrediidae.—Shell donaciform, ligament external, cardinals usually two in each valve, posterior laterals strong. Trias——. Genera: Tancredia (Secondary strata), Hemidonax.
[454]
Fam. 5. Cardiliidae.—Shell heart-shaped, hinge as in Mactridae, posterior adductor resting on a myophore or shelf. Single genus, Cardilia. Tertiary——.
Fam. 6. Mesodesmatidae.—Mantle edges largely united, with three orifices, foot byssiferous or not; shell regular or irregular, usually one cardinal and strong lateral teeth. Tertiary——. Genera: Mesodesma, Ervilia.
Fam. 7. Mactridae.—External branchial fold directed ventrally, siphons fused, foot tongue-shaped; shell equivalve, triangular-oval, hinge with ligament in an internal fossette, another portion external, a bifurcated cardinal tooth in the left valve, fitting into a branching tooth in the right valve, laterals present. Jurassic——. Genera: Nactra, Harvella, Raëta, Eastonia, Heterocardia, Vanganella.
Sub-order III. Veneracea.—Branchiae slightly folded, foot compressed, siphons generally short, pallial line variable, two adductor muscles.
Fam. 1. Veneridae.—Siphons free or partly united, foot seldom byssiferous; shell solid, equivalve, hinge usually with three cardinal teeth, laterals variable. Jurassic——. Principal genera: Cytherea, Circe; Grateloupia (Tertiary), Meroe, Dosinia (= Artemis), Cyprimeria, Cyclina, Venus, Clementia, Lucinopsis; Thetis (Cretaceous), Tapes, Venerupis.
Fam. 2. Petricolidae.—Animal perforating rocks; shell oval, slightly gaping behind, two or three cardinals, no laterals, pallial sinus well marked. Recent——. Genera: Petricola, Naranio.
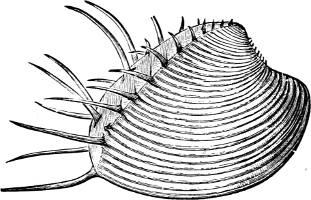
Fig. 307.—Cytherea dione Lam., Peru.
Fam. 3. Glaucomyidae.—Siphons long, united, foot small; shell produced, thin, hinge with three cardinals, no laterals, pallial sinus well marked. Recent——. Genus, Glaucomya (incl. Tanysiphon).
Sub-order IV. Cardiacea.—Branchiae much folded back, mantle edges with three apertures, foot cylindroidal, more or less produced, siphons present or absent, one or two adductor muscles, pallial line variable.
Fam. 1. Cardiidae.—Siphons rather long, foot long, no byssus; shell equivalve, more or less radiately ribbed, hinge with[455] one or two cardinals in each valve, laterals variable, ligament external, two adductors. Brackish water or marine. Devonian——. Genera: Byssocardium and Lithocardium (Tertiary), Conocardium (Palaeozoic), Cardium (with many sections, including Hemicardium), Limnocardium (subg. Didacna, Monodacna, Adacna).
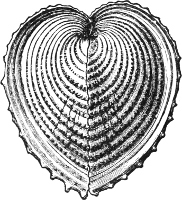
Fig. 308.—Cardium (Hemicardium) cardissa L., East Indies.
Fam. 2. Lunulicardiidae.—Shell equivalve, very inequilateral subtriangular, anterior margin short or truncated, with a deep lunule. Single genus, Lunulicardium (Palaeozoic).
Fam. 3. Tridacnidae.—Mantle orifices widely separated, foot short, byssiferous, no anterior adductor; shell equivalve, large, thick, usually gaping in front, one cardinal tooth and one or two posterior laterals in each valve, no pallial sinus. Miocene——. Genera: Tridacna, Hippopus. The muscular power of the great Tridacna is immense. Once caught between their gaping valves, a man’s hand or foot can scarcely be withdrawn. Two valves of T. gigas in the British Museum weigh respectively 154 and 156 lbs.
Fam. 4. Chamidae.—Mantle orifices widely separated, foot short, no byssus, both adductors present, ovary invading the mantle lobes; shell fixed, irregularly inequivalve, umbones spiral, ligament external, cardinal teeth often a mere ridge, anterior lateral strong, nearly central, no pallial sinus. Jurassic——. Genera: Chama; Diceras (Jurassic), attached by one umbo, umbones very prominent, teeth strong; Heterodiceras (Jurassic), Requienia (Cretaceous), left valve widely spiral, attached by the umbo, right valve small, fitting on the other as an operculum, teeth obsolete; Toucasia, Apricardia, Matheronia (all Secondary strata).
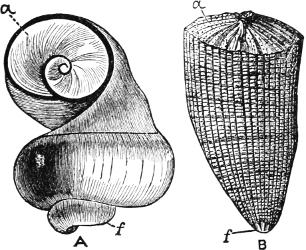
Fig. 309.—A, Requienia ammonea Goldf., Neocomian, × ½; B, Hippurites cornu-vaccinum Goldf., Cretaceous, × ¼. a, right valve; f, point of fixture. (From Zittel.)
[456]
The four succeeding families require special study in a work on Palaeontology.
Fam. 5. Monopleuridae.—Shell very inequivalve, left valve operculiform, right conical or spiral, fixed at the apex, ligament prolonged in external grooves. Cretaceous——. Genera: Monopleura, Valletia.
Fam. 6. Caprinidae.—Shell very inequivalve, thick, free or fixed by apex of right valve, which is spiral or conical, left valve spiral or not, often perforated by radial canals from the umbo to the free margin. Neocomian and Cretaceous——. Principal genera: Plagioptychus, Caprina, Ichthyosarcolites, Caprotina, Polyconites.
Fam. 7. Hippuritidae (= Rudistae).—Shell very inequivalve, externally as in Caprinidae, umbo central in left valve, no ligament proper, left valve with strong hinge-teeth and grooves, two adductor impressions on prominent myophores, shell structure of the two valves differing. Cretaceous only. Single genus, Hippurites (Fig. 309, B).
Fam. 8. Radiolitidae.—Shell inversely conical, biconical, or cylindrical, general aspect of Hippurites, umbo of left valve central or lateral, right valve with a thick outer layer, often foliaceous, umbonal cavity partitioned off by laminae. Cretaceous only. Genera: Radiolites, Biradiolites.
Sub-order V. Myacea.—Branchiae much folded back, mantle edges usually with three openings, foot compressed, siphons large, united or not, two adductor muscles, pallial line variable.
Fam. 1. Psammobiidae.—Siphons long, not united, foot large, not byssiferous; shell equivalve, long, oval, slightly gaping at the ends, ligament external, prominent, two cardinal teeth in each valve, no laterals, a deep pallial sinus. Jurassic——. Genera: Psammobia, Solenotellina, Sanguinolaria, Asaphis, Elizia, Quenstedtia (Jurassic).
Fam. 2. Myidae.—Pedal orifice small, siphons long, united in great part; shell inequivalve, gaping at one or both ends, periostracum more or less extensive, ligament internal, resting on a prominent shelf; hinge-teeth variable. Cretaceous——. Genera: Mya, Tugonia, Sphenia, Corbula, Lutraria (for which latter some propose a separate family).
Fam. 3. Solenidae.—Foot long, powerful, more or less cylindrical, no byssus, siphons usually short, united or not, branchiae[457] narrow; shell equivalve, long and narrow, gaping at both ends, with periostracum, umbones flattened, ligament external, hinge-teeth variable.? Devonian——. Genera: Solecurtus, Pharella, Pharus, Cultellus, Siliqua, Ensis, Solen, Orthonota (?), Palaeosolen (?).
Fam. 4. Glycimeridae.—Pedal orifice very narrow, siphons long, united in great part, often covered with periostracum; shell more or less equivalve, gaping at both ends, hinge toothless or with two weak cardinals, ligament external; animal free or perforating. Cretaceous——. Genera: Glycimeris, Saxicava, Cyrtodaria.
Fam. 5. Gastrochaenidae.—Foot small, cylindrical, no byssus, branchiae narrow, siphons long; shell perforating or cemented to a shelly tube, gaping widely on the anterior and ventral sides, no hinge-teeth, a deep pallial sinus. Cretaceous——. Genera: Gastrochaena, Fistulana (tube with a median diaphragm, perforated by the siphons).
Sub-order VI. Pholadacea.—Mantle edges largely closed, siphons long, united, foot short, truncated, disc-shaped, ligament absent, two adductor muscles; animal perforating.
Fam. 1. Pholadidae.—Organs contained within the valves, ctenidia prolonged into the branchial siphon, shell more or less gaping, thin, dorsal margin in part reflected over the umbones, one or more dorsal accessory pieces, no hinge-teeth, an interior apophysis proceeding from the umbonal cavity. Jurassic——. Genera: Pholas, Talona, Pholadidea (posterior extremity of the valves prolonged by a corneous appendage, a passage to the long tube of Teredo), Jouannetia, Xylophaga, Martesia; Teredina (Eocene).
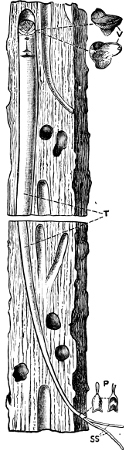
Fig. 310.—Teredo navalis L.: V, valves of shell; T, tube; P, pallets; SS, siphons. (After Möbius.)
Fam. 2. Teredinidae.—Animal vermiform, ctenidia mainly[458] within the branchial siphon, siphons very long, with two calcareous appendages (“pallets”) near the anterior end, shell very small, continued into a long calcareous tube, valves deeply notched, internal apophysis as in Pholadidae. Lias——. Single genus, Teredo (Fig. 310).
Sub-order VII. Anatinacea.—External branchial fold directed dorsally, not reflected, sexes united, ovaries and testes with separate orifices, mantle edges largely united, byssus usually absent, two adductor muscles, pallial line variable, shell usually nacreous within.
Fam. 1. Pandoridae.—Siphons short, largely united, foot tongue-shaped; shell free or fixed, inequivalve, semi-lunar, or subtriangular, ligament often with calcareous ossicle, pallial line complete or with slight sinus. Cretaceous——. Genera: Pandora, Myodora, Myochama.
Fam. 2. Chamostreidae.—Shell fixed, Chama-like, thick, umbones spiral, ligament with ossicle. Single genus, Chamostrea.
Fam. 3. Verticordiidae.—Siphons not prolonged; shell heart-shaped, umbones prominent, spiral, ligament with an ossicle, pallial line complete. Miocene——. Genera: Verticordia, Mytilimeria, Lyonsiella.
Fam. 4. Lyonsiidae.—Foot short, byssiferous, siphons short, separate, shell inequivalve, hinge-teeth usually absent, ligament and ossicle in an internal groove. Eocene——. Single genus, Lyonsia.
Fig. 311.—Myochama Stutchburyi A. Ad., attached to Circe undatina Lam., Moreton Bay.
Fam. 5. Ceromyidae.—Shell inequivalve, large, heart- or wedge-shaped, hinge toothless, ligament internal in one valve, external in the other. Secondary strata——. Genera: Ceromya, Gresslya.
Fam. 6. Arcomyidae.—Shell equivalve, thin, surface finely granulated, hinge toothless, cardinal edge dentiform, ligament external. Secondary and Tertiary strata——. Genera: Arcomya, Goniomya, Pleuromya, Machomya.
Fam. 7. Anatinidae.—A fourth (?byssal) pallial orifice, siphons long, separate or fused; shell thin, sometimes inequivalve,[459] exterior often granulose, ligament often with ossicle, hinge toothless or with lamellae. Jurassic——. Genera: Anatina, Plectomya (Secondary), Periploma, Cochlodesma, Thracia, Tyleria, Alicia, Asthenothaerus.
Fam. 8. Grammysiidae.—Shell equivalve, oval, ligament external, cardinal margin straight, toothless, pallial line complete. Palaeozoic——. Principal genus, Grammysia, with many other genera of toothless hinge, but whose exact affinities are uncertain.
Fam. 9. Praecardiidae.—Shell thin, equivalve or not, radiately ribbed, margins dentated, sub-umbonal area as in Arca, hinge toothless. Palaeozoic——. Principal genus, Praecardium.
Fam. 10. Pholadomyidae.—A fourth pallial orifice, siphons very long, united, foot small; shell thin, equivalve, with radiating ribs, ligament external, hinge toothless, pallial line sinuate. Jurassic——. Single genus, Pholadomya.
Fam. 11. Clavagellidae.—Foot rudimentary, siphons long, united, contained in a long calcareous tube; shell small, one or both valves soldered in the tube, tube with a centrally fissured disc at the anterior end, more or less frilled at the posterior end. Cretaceous——. Genera: Clavagella, Brechites (= Aspergillum).
Mantle edges united at three points, branchiae replaced by a muscular septum extending from the anterior adductor to the point of separation of the siphons, septum with symmetrical orifices.
Fam. 1. Poromyidae.—Branchial septum with groups of lamellae between the orifices, labial palps large, foot long and narrow, siphons short, papillose, separated, animal hermaphrodite; shell small, slightly inequivalve, rounded or oblong, nacreous within. Eocene——. Genera: Poromya, Silenia.
Fam. 2. Cuspidariidae.—Siphons long, united in part, foot short, animal dioecious; shell small, slightly inequivalve, rostrate, not nacreous, each valve with ligamentary cartilage spoon-shaped, with a calcareous ossicle, cardinal and lateral teeth present or absent. Jurassic——. Single genus, Cuspidaria, with many sections.
[461]
PART I
RECENT BRACHIOPODA
BY
ARTHUR E. SHIPLEY, M.A.
Fellow and Tutor of Christ’s College, Cambridge
[463]
INTRODUCTION—SHELL—BODY—DIGESTIVE SYSTEM—BODY CAVITY—CIRCULATORY SYSTEM—EXCRETORY ORGANS—MUSCLES—NERVOUS SYSTEM—REPRODUCTIVE SYSTEM—EMBRYOLOGY—HABITS—DISTRIBUTION—CLASSIFICATION
The group Brachiopoda owes its chief interest to the immense variety and great antiquity of its fossil forms. Whereas at the present time the number of extant species amounts to but about 120, Davidson in his admirable monograph[410] on the British Fossil Brachiopoda has enumerated close upon 1000 fossil species, found within the limits of the United Kingdom alone.
The amount of interest that the group in question has excited amongst naturalists is evinced by the invaluable Bibliography of Brachiopoda, prepared by the same author and his friend W. H. Dalton.[411] This monument of patient research contains over 160 quarto pages, each with the titles of from eighteen to twenty separate papers dealing with Brachiopods, published between the years 1606 and 1885.
Probably the first reference to Brachiopods in zoological literature is to be found in a work entitled Aquatilium et Terrestrium aliquot Animalium, published in the year 1606 by Prince Fabio Colonna at Rome. This work contains the first description of a Brachiopod under the name of Concha diphya. In a second edition, which is not so rare in our libraries as the[464] first, the author mentions three more species of Brachiopods. Towards the end of the same century, Martin Lister of Oxford, in his Historia sive Synopsis methodica Conchyliorum which appeared in parts, described and figured a considerable number of Brachiopods, which, under the name of Anomia, were until the present century regarded as Molluscs, and placed in the subdivision Pelecypoda (Lamellibranchiata).
The first satisfactory figure and description of a Terebratula were published in the year 1766, in Pallas’ Miscellanea Zoologica, still under the name Anomia. In 1781 O. F. Müller figured a Crania, under the name Patella anomala, the generic name being subsequently altered by Cuvier into Orbicula.
Bruguière in the year 1789 was the first to recognise the relationship between Lingula and the other Brachiopods. He for the first time saw the stalk of this genus, and compared it with that of the stalked Barnacles, a class of animals which has been more than once associated with our group.
Cuvier in his Mémoire sur l’Anatomie de la Lingule, 1797, gave the first account of the internal anatomy of a Brachiopod. The same naturalist first described the nephridia, although his mistake in considering them lateral hearts was not rectified until the middle of the present century, when Huxley pointed out that these structures serve as excretory ducts for the genital products.
Duméril in 1807 proposed the somewhat unfortunate name of Brachiopoda; and although efforts have been made by de Blainville, who suggested Palliobranchiata, and more recently by Haeckel, who proposed Spirobranchiata, to arrive at a name which would be both grammatically and physiologically more correct, the older name has maintained its position, and is now universally in use.
In 1834 and 1835 Professor Owen published the results of his researches into the anatomy of the Brachiopoda. He investigated in these years the structure of Waldheimia flavescens, of a species of Lingula and of a Discina, called by him Orbicula. He regarded the group as midway between the Pelecypoda and the Ascidians. The structure of Lingula was further investigated by Carl Vogt, who in 1851 also supported the view that the Brachiopoda were related to the Mollusca. But already in 1847 and 1848 Steenstrup had thrown doubts upon this relationship, and had maintained that the Order was more closely related[465] to certain members of the Chaetopoda, a view which afterwards found its ablest supporter in the American naturalist Morse.
D’Orbigny seems to have been the first observer who drew attention to the resemblances alleged to exist between the Brachiopoda and the Polyzoa, and Hancock, in his masterly works On the Anatomy of the Fresh-water Bryozoa (Polyzoa) and in his Organisation of the Brachiopoda, dwelt on these resemblances, and placed the Brachiopoda between the Polyzoa on the one hand and the Ascidians on the other; a collocation which subsequently resulted in their inclusion in the now discarded group of Molluscoidea.
In 1854 Huxley[412] published what is, with the possible exception of Hancock’s monograph, mentioned above, the most important work upon the anatomy of the Brachiopoda with which we are acquainted. He corrected numerous errors of his predecessors and added many new facts to our knowledge of the group. He was the first to describe the true nature of the lateral hearts of Cuvier, and to describe the true heart, afterwards so carefully figured by Hancock.
A further step was made in 1860 and 1861 by the discovery and description of the larvae of Brachiopoda, by F. Müller and Lacaze-Duthiers. Since that time we owe what little advance has been made in the embryology of the group to the researches of Morse and of Kowalevsky.
Modern methods of research—section cutting, etc.—were first applied to the group by the Dutch naturalist, van Bemmelen,[413] from whose admirable historical account of our knowledge of the group many of the above facts have been gathered. These methods have thrown considerable light upon the histology of the group, but have not added very much to our knowledge of the structure or the affinities of the Brachiopoda. The modern views as to the latter point may be best discussed after some account of the anatomy of the various genera has been given.
The Shell
The body of a Brachiopod is enclosed within a bivalve shell, but the two halves are not, as they are in the Pelecypoda,[466] one on each side of the body, but occupy a different position with regard to the main axes of the body. What this position is, has formed the subject of a good deal of discussion. For our purpose, however, it will suffice to distinguish the two valves by the most commonly accepted terms of dorsal and ventral. The former is, as a rule, the smaller of the two, and usually lies on the lower surface of the animal in life. Adopting the orientation indicated above, the stalk by means of which the Brachiopoda are attached to the rocks and stones, etc., upon which they live, becomes posterior, and the broader edge of the two shells, which are capable of being opened to some extent, is anterior.
Fig. 312.—Four specimens of Terebratulina caput serpentis, attached to a waterlogged piece of wood, from the Clyde area.
The posterior end of the shell usually narrows, and the ventral valve projects behind the dorsal, and may be produced into a sort of beak or funnel, through the aperture of which the stalk protrudes. This aperture may be completed by the ventral shell, or the latter may only be notched, in which case the hole is completed by the posterior edge of the dorsal shell.
The nature of the shell has been used in classifying the group into two orders:—
I. The Ecardines, whose shell is chitinous but slightly strengthened by a deposit of calcareous salts. There is no hinge and no internal supports for the arms. The alimentary canal terminates in an anus.
II. The Testicardines, whose shells are composed of calcareous spicules. The valves are hinged together, and there is usually an internal skeleton supporting the arms. There is no anus.
The outside of the shell of recent Brachiopods is often smooth, but many are ridged. In a recent species, Rhynchonella Döderleini from Japan, Davidson[414] has described a number of spines[467] arranged in concentric circles on the ribbed shell. They are not so long as the spines irregularly scattered on the shell of Rh. spinosa from the Jurassic formations. Some shells are brightly coloured, as, for instance, the various species of Cistella which live on the coralline rock in the Mediterranean; these exhibit bands or rays of alternate orange and bright pink. On the other hand, the shells of Terebratula vitrea are of a slightly translucent white, and of the utmost delicacy. They are very large, so that the cavity within the valves is of much greater size than the body of the animal, but in other genera the soft parts are packed very closely, and there is but a very small mantle-cavity or space within the shell unoccupied by the body of the animal. It is, however, more common for the shells of Brachiopods to be of a dull yellowish colour, and to be somewhat massive. Most species are attached by a pedicle or stalk to some rock or stone at the bottom of the sea, but in some, as in Crania, the ventral valve becomes closely adherent to its support, so much so that it is difficult, or impossible, to remove the animal without leaving the ventral valve behind. Lingula, like Crania, one of the Ecardines, lives in sand (Fig. 321, p. 483), and does not use its long pedicle to adhere to any fixed object. . The outline of the shell varies extremely. It may be almost round or prolonged along either axis; the edges of the valves may be smooth or fluted in correspondence with the ridges and grooves of the outside of the shell.
Fig. 313.—Three specimens of Crania anomala on a stone dredged in Loch Fyne. The topmost specimen is seen in profile.
On the inner surface of the shell of the Testicardinate Brachiopoda, at the hinder end of the ventral valve, are two lateral teeth, which fit into corresponding sockets in the dorsal valve. These form a hinge, which in many cases is so arranged as to permit the shell to be opened to only a very limited extent. There are also certain plate-like processes which project into the lumen of the shell, and help to support various portions of the body; and in Terebratula, Waldheimia, etc., these form a complicated band-like loop, which increases in complexity with advancing age, and serves to support the arms. In the extinct[468] Spirifera the internal skeleton takes the form of two spirally coiled lamellae, which almost entirely fill the cavity of the shell; the apices of the spirals point outwards (Fig. 330). The inner surface of the shell also bears the marks of the insertion of the numerous muscles which govern its movements.
Microscopic examination of thin sections of the shell shows that it consists of small prisms or spicules of calcareous substance, whose long axis lies, roughly speaking, at right angles to the surface of the shell. These spicules are held together by an organic matrix, in which, however, no cellular elements can be detected. In sections made through a decalcified shell the position of the spicules which have been dissolved by the acid is indicated by spaces, and the matrix remains as a network of fibrils, which end on the outside in a thin cuticular layer of organic matter. In Lingula and Discina the organic matter takes a much larger share in the formation of the shell, which in these genera consists of a number of alternating layers of horny and calcareous matter. The former is described by Gratiolet as fibrillated, the fibrils being obliquely placed, whilst the latter consists of a number of small prisms set at right angles to the surface of the shell.
In many genera, as in Terebratula, Terebratella, Cistella, Waldheimia, Crania, etc., the shell is pierced by a number of small canals (Fig. 314), which in the dried specimens form so many open pores, but in the living animal contain prolongations of the mantle or body wall which secretes the shell. They contain extensions of the layer of epithelial cells which lines and secretes the shell. The canals come to the surface and at their outer end are often slightly swollen. They are closed by the cuticular layer which is mentioned above as covering the shell externally. They are not found in the loops or other internal processes of the shell. In Crania the canals depart to some extent from the usual type; instead of running a straight course to a somewhat swollen outer end, they break up into a number of very fine branching tubules, which form a very minute meshwork near the surface of the shell. These fine branches contain protoplasmic fibrils, which have their origin in the epithelial cells which lie in the tubules.
By carefully counting the number of tubules in a given area of young and old specimens of Waldheimia cranium, van Bemmelen[415][469] was able to show that the spaces between the tubules did not increase with age. He therefore reasoned that the shells of Brachiopoda do not increase by intussusception, and that their increase in size must be entirely due to additions made round their free edge.
The function of the tubules has been a matter of some discussion. They have been regarded as respiratory organs, but it would seem more reasonable to suppose that they serve as organs to supply nourishment, etc., to the organic matrix of the shell.[416]
With the exception of the genus Crania, it is usual for Brachiopods to bear round the edge of their mantle rows or bundles of chitinous setae or bristles (Figs. 315 and 319). The length and arrangement of these structures vary in the different species; they are secreted from little pits in the edge of the mantle. It seems probable that they serve to some extent as organs of defence, especially in the larva, where they make their appearance at an early stage; possibly they also serve as a filter, and prevent the entrance of foreign bodies into the shell. Their presence has been taken to indicate a certain degree of affinity between Brachiopods and Chaetopods, since setae are very characteristic of the last-named group.
The shell of a Brachiopod is secreted partly by the general surface of the body which is situated at the hinder end of the shell, and partly by the two leaf-like extensions of the body, which are termed the dorsal and ventral mantles. These are, in fact, folds of the body wall, and into them the body cavity and certain of its contents, such as the liver and generative glands, etc., extend. The space between the two folds of the mantle, which is limited behind by the anterior wall of the body, is termed the pallial or mantle cavity. On each side of the middle line the anterior wall of the body is produced into two “arms,” which occupy as a rule a considerable part of the mantle cavity. These arms may be but flattened portions of the general body wall, which occupies a large part of what in[470] other genera is the mantle of the dorsal valve, as in Cistella and Argiope;[417] or they may be outgrowths of the body wall in the form of long processes, which are coiled and twisted in a very characteristic manner in the various genera. In any case the cross section of the arm shows a groove, one side of which forms a continuous lip, and the other takes the form of a single row of tentacles, which are richly ciliated and capable of considerable movement. The whole arm in Rhynchonella can be protruded from the shell, as was noted years ago by O. F. Müller, and although his statement to this effect has often been doubted, its truth was confirmed by Professor Morse,[418] who writes: “In the year 1872, while studying living Rhynchonella in the St. Lawrence, I observed a specimen[471] protrude its arms to a distance of 4 c.m. beyond the anterior borders of the shell, a distance nearly equalling twice the length of the shell.” The same observer also mentions that Lingula has the power of partially protruding its arms. In most genera the cirrhi or tentacles can alone be protruded.
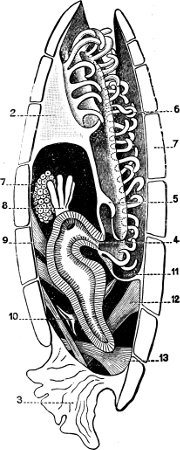
Fig. 314.—View of the left half of Cistella (Argiope) neapolitana, which has been cut in two by a median longitudinal incision, to show the disposition of the organs. Partly diagrammatic. The inorganic part of the shell only is shown. The tubular extensions of the mantle and the organic outer layer are not indicated, and hence the pores appear open.
The cilia which clothe the tentacles keep up a constant flow of water into the mantle cavity. This stream not only serves to aerate the blood of the animals—a process which probably takes place through the thin inner lining of the mantle—but it also brings with it a number of diatoms and other minute organisms which serve as food. These particles become entangled in the tentacles, and are ultimately lodged in the groove at their base, and passing along this by the action of the cilia they find their way into the wide mouth, into which the groove deepens in the posterior median line.
The mouth leads into an oesophagus; this widens into a chamber which may be termed the stomach (Fig. 314), and which receives the openings of two large branching glands usually known as the liver. The stomach passes into a short intestine which is usually bent at about a right angle with the oesophagus. In the Testicardines the intestine ends blindly, but in the Ecardines it is of much greater length, and terminates in an anus, situated posteriorly in the median line in Crania, but asymmetrical and to the right of the body in Lingula (Fig. 315) and Discina; in both cases, however, the opening is into a portion of the mantle cavity. The alimentary canal is supported by a median dorsal and ventral mesentery, and by two pairs of lateral mesenteries which pass to the body wall. The lateral mesenteries are not always quite distinct. When they are, the anterior pair are known as the gastro-parietal bands, and the posterior as the ileo-parietal. In Rhynchonella there are two pairs of renal organs, and each of these mesenteries bears the internal openings of one pair. In all other Brachiopods there is only one pair, and they are supported by the ileo-parietal bands.
The alimentary canal is ciliated throughout, and some interesting observations have been made by Schulgin[419] on the shortening[472] of these cilia in Argiope (Cistella) when the animal is well fed, and their elongation when the animal is hungry. Amongst the ciliated cells certain glandular cells have been described. The so-called liver consists of two more or less branching glands, which open by wide apertures, one on each side of the stomach. It seems probable that a good deal of digestion is carried on in these glands, since the diatoms and other minute organisms upon which the Brachiopoda live are usually found in the branches of these glands, and the glandular cells lining the tubules vary much in appearance according to the animal’s state of nutrition.
The alimentary canal and liver occupy a considerable portion of the body cavity or general space of the body; this space is to some extent cut up by the various mesenteries above mentioned. It also lodges the reproductive organs and the excretory ducts. Its walls are ciliated, and the action of the cilia keeps in motion the corpusculated fluid that bathes the various organs in the body cavity. The mantles, which are nothing but flattened leaf-like extensions of the body wall lining the shell, also contain diverticula of the body cavity, which may be simple flattered spaces or may be broken up into definite channels, as in Lingula (Fig. 315). It seems not improbable that the body cavity fluid is aerated through the thin inner layer of the mantle.
Fig. 315.—View of the inner side of a valve of Lingula anatifera (after François), to show the definite arrangement of the channels in the mantle: a, position of mouth; b, position of anus.
Running along the base of each arm are two canals, a small one at the base of the tentacles, which we may term the tentacular canal, and a larger one, the canal of the lip. The former sends a prolongation into each tentacle. The latter is, according to Blochmann, a closed canal in Crania, Lingula, Rhynchonella,[473] and others; but according to Joubin,[420] it communicates in Crania at one point with the tentacular canal. It is probably originally a part of the body cavity. Blochmann[421] states in very definite terms that in Crania neither the large canal nor the small canal communicates with the general body cavity, but he admits that in Lingula the small canal opens into that space.
The details of the discovery of the central circulatory organ of Brachiopods form a curious and instructive chapter in the history of modern morphological inquiry. Hancock, in his monograph on the group, described and figured on the dorsal surface of the alimentary canal a well-developed heart, which had been previously noticed by Huxley, who first showed that the organs which up to his time had been regarded as hearts were in reality excretory organs. In connexion with this heart Hancock described numerous arteries, distributed to various parts of the body. The observers who have written upon the anatomy of Brachiopods since Hancock’s time, in spite of the fact that they had at their disposal such refined methods of research as section cutting, which was quite unknown at the time his monograph was written, have almost all failed to find this circulatory system, and many of them have been tempted to deny its existence. Blochmann,[422] however, in the year 1885 stated that he had found the heart, and had seen it pulsating in several species of Brachiopoda which he had rapidly opened whilst alive. Joubin has also described it in large specimens of Waldheimia venosa, and recently Blochmann has published a detailed account of his work on this subject. Both these authors describe the heart as a vesicle with muscular walls, situated dorsal to the alimentary canal. From this, according to Blochmann, a vessel—the branchio-visceral of Hancock—runs forward as a triangular split in the dorsal mesentery supporting the alimentary canal. This vessel divides into two at the oesophagus, and passing through some lacunae in the walls of this[474] tube, opens into the tentacular canal, and consequently supplies the tentacles with blood. These two canals which diverge from the median artery are connected ventrally by a vessel which runs below the oesophagus; the latter is therefore surrounded by a vascular ring. Blochmann also describes two pairs of vessels that were seen and figured by Hancock. A pair of these pass over the gastro-parietal mesenteries and into the dorsal mantle sinus, the second pair pass over the ileo-parietal mesenteries and into the ventral mantle sinus; each of these four arteries runs to one of the four generative glands, which, as is so usually the case in the animal kingdom, have thus a specially rich blood supply. If this description should prove to be correct, the vascular system of Brachiopods shows a striking resemblance to that of the closed vascular system of the unarmed Gephyrea, except that the former group has specialised genital vessels. The blood is colourless.
Joubin’s description of the vascular system of W. venosa differs in some respects from that of Blochmann. He regards the heart as collecting the lymph which it receives from numerous lacunar spaces in the walls of the alimentary canal, and distributing it through various vessels, which in the main correspond with those of Blochmann, and which run both to the “arms” and to the generative glands. The latter vessels, however, open freely into the body cavity, and the fluid which is forced out from their openings freely bathes the organs found in the body cavity. Whichever of these accounts should prove to be more closely in accordance with the facts, there is little doubt that in addition to the true blood there is a corpusculated fluid in the body cavity which is to some extent kept in motion by the ciliated cells that line its walls.
The excretory organs (kidneys) which were at one time regarded by Cuvier and Owen as hearts, are typical nephridia—that is to say, they are tubes with glandular excretory walls which open at one end by a wide but flattened funnel-shaped opening into the body cavity, and at the other end by a circular pore to the exterior (Fig. 314). In Rhynchonella, where there are two pairs of these tubes,—the only evidence that the group presents of any metameric repetition of parts,—the inner[475] ends of the anterior pair are supported by the gastro-parietal mesenteries, and those of the posterior pair by the ileo-parietal mesenteries. In all other Brachiopods the posterior pair alone exists. The external opening of these nephridia is near the base of the anus; in Cistella it is at the bottom of a brood-pouch formed by the tucking in of the body wall in this neighbourhood, and in this brood-pouch the eggs develop until the larval stage is reached.
The walls of these nephridia are lined by ciliated cells, amongst which are some excretory cells, in which numerous brown and yellow concretions are to be seen; these are probably the nitrogenous excreta of the animal, and pass out of the body, being washed away by the stream of water which is constantly passing between the shells.
As in so many other animals, the nephridia act as genital ducts, and through them the ova and spermatozoa, which break off from the genital glands and fall into the body cavity, find their way to the outer world.
The body cavity of a Brachiopod is traversed by several pairs of muscles, which are very constant in position, and whose contraction serves to open and close the shell, to move the animal upon its stalk, and to govern the movements of the arms.
The stalk is absent in Crania, and the members of this genus are attached to the rocks on which they are found by the whole surface of their ventral valve. In Lingula (Fig. 315) the stalk is long and hollow, containing what is probably a portion of the body cavity, surrounded by muscular walls. Lingula is not a fixed form, but lives half-buried in the sand of the sea-shore (Fig. 321). Discina, the other member of the Ecardines, has a peduncle which pierces the ventral valve and fixes the animal to its support. Amongst the Testicardines, Thecidium is also fixed to its supports by the surface of its ventral valve; the other genera, however, are provided with stalks, which, being the means of the fixation of the animals, become at the same time the fixed points upon which their very limited movements can be effected. The stalk protrudes through the notch or aperture at the posterior end of the ventral[476] valve, and it probably belongs to the ventral side of the body. It is in Cistella, and doubtless in other genera, in close organic connexion with both valves, and it seems to consist of an unusually large development of the supporting tissue which occurs so frequently in the body of Brachiopods. The surface of the peduncle is produced into several irregularities and projections which fit into any depressions of the rock upon which the animal is fixed.
In Cistella there are four pairs of muscles, two connected with opening and closing the shell, and two with the movement of the body upon the stalk (Fig. 314). The most considerable of these muscles are the two occlusors, which have their origin, one on each side of the middle line of the dorsal valve, and their insertion by means of a tendon into the ventral valve. In the species in question each of these muscles arises by a double head, the two muscles thus formed probably representing the anterior and posterior occlusors of other forms. The contraction of these muscles undoubtedly serves to close the shell, which is opened by a small pair of divaricators arising from the ventral valve, and inserted into a portion of the dorsal shell which is posterior to the axis of the hinge. Contraction of these muscles would thus serve to approximate the posterior edges of the valves and divaricate the anterior edges and thus to open the shell.
The adjustors are four in number, a ventral pair running from the ventral valve to be inserted into the stalk, and a corresponding dorsal pair from the dorsal valve. The simultaneous contraction of either pair would tend to raise the valve, whilst the alternate contraction of the muscles of each side would tend to rotate the shell upon the peduncle. The muscles of Waldheimia flavescens are shown in Fig. 329, and described briefly on p. 502.
The muscles of the Ecardines differ from those of the Testicardines inasmuch as they do not terminate in a tendon, but the muscle fibres run straight from shell to shell. They are also more numerous. In Crania there is an anterior and a posterior pair of occlusor muscles, and two pairs of oblique muscles, which seem when they contract together to move the dorsal shell forwards, or when they contract alternately to slightly rotate it. In this genus there are also a pair of protractors[477] and a pair of retractors, and two levators of the arms, whose function is to draw forward or retract the arms, and an unpaired median or levator ani muscle. In addition to these bundles of muscles there are certain muscles in the body wall, and it seems probable that by their contraction, when the adductors are relaxed, the body may become somewhat thicker and the valves of the shells will slightly open.
Fig. 316.—A semi-diagrammatic figure of the muscular system of Crania (after Blochmann): a, anterior occlusor; b, posterior occlusor; c, superior oblique; d, inferior oblique; e, retractor of the arms; f, elevator of the arms; g, protractor of the arms; h, unpaired median muscle. The dorsal valve is uppermost.
In Lingula (Fig. 322) the muscular system is more complicated; in addition to the anterior (= anterior laterals) and posterior (= centrals) pairs of occlusors, there is a single divaricator (= umbonal), whose contractions in conjunction with those of certain muscles in the body wall press forward the fluid in the body cavity, and thus force the valves of the shell apart; and there are three pairs of adjustor muscles. These latter are called respectively the central (= middle laterals), external (= external laterals), and posterior (= transmedians) adjustors, whose action adjusts the shells when all contract together, and brings about a certain sliding movement of the shells on one another when they act independently of each other.[423]
[478]
The nervous system of Brachiopods is not very clearly understood, and there are considerable discrepancies in the accounts of the various investigators, even when they are dealing with the same species. So much, however, seems certain, that there is a nervous ring surrounding the oesophagus, that this ring is enlarged dorsally, or, in other words, near the base of the lip, into a small and inconspicuous dorsal ganglion, and again ventrally or just behind the base of the tentacles into a ventral or sub-oesophageal ganglion. The latter is, contrary to what is usual in Invertebrates, of much larger size than the supra-oesophageal ganglion, but like the last named, it has retained its primitive connexion with the ectoderm or outermost layer of the skin. Both ganglia give off a nerve on each side which runs to the arms and along the base of the tentacles and lips. The sub-oesophageal ganglion also gives off nerves which supply the dorsal and ventral folds of the mantle, the muscles, and other parts.
The modified epithelium in connexion with the ganglia may possibly have some olfactory or tactile function, but beyond this the Brachiopoda would appear to be devoid of eyes, ears, or any other kind of sense organs,—a condition of things doubtless correlated with their sessile habits, and with the presence of a bivalved shell which leaves no part of their body exposed.
The majority of Brachiopods are bisexual, and many authorities regard the separation of sex as characteristic of the group; on the other hand, Lingula pyramidata is stated to be hermaphrodite, and it is not impossible that other species are in the same condition.
The generative organs are of the typical sort, that is, they are formed from modified mesoblastic cells lining the body cavity. These cells are heaped up, usually in four places, and form the four ovaries or testes as the case may be (Fig. 314). The generative glands usually lie partly in the general body cavity and partly in the dorsal and ventral mantle folds, two on each side of the body. Along the axis of the heaped-up cells[479] runs a blood-vessel, which doubtless serves to nourish the gland, the outer surface of which is bathed in the perivisceral fluid. Every gradation can be found between the ripe generative cell and the ordinary cell lining the body cavity. When the ova and spermatozoa are ripe they fall off from the ovary and testis respectively into the body cavity, thence they are conveyed to the exterior through the nephridia. The ova in certain genera, such as Argiope, Cistella, and Thecidium, develop in brood-pouches which are either lateral or median involutions of the body wall in the neighbourhood of the external opening of the nephridia; they are probably fertilised there by spermatozoa carried from other individuals in the stream of water which flows into the shell. In other species the ova are thrown out into the open sea, and their chances of meeting with a spermatozoon is much increased by the gregarious habits of their sessile parents, for as a rule considerable numbers of a given species are found in the same locality.
We owe what little we know of the Embryology of the group chiefly to Kowalevsky,[424] Lacaze-Duthiers,[425] and Morse.[426] The Russian naturalist worked on Cistella (Argiope) neapolitana, the French on Thecidium, and the American chiefly on Terebratulina.
Although this is not known with any certainty, it seems probable that the eggs of Brachiopods are fertilised after they have been laid, and not whilst in the body of the mother. The spermatozoa are doubtless cast out into the sea by the male, and carried to the female by the currents set up by the cilia clothing the tentacles.
In Thecidium, Cistella, and Argiope the first stages of development, up to the completion of the larva, take place in brood-pouches; in Terebratulina the eggs pass out of the shell of the mother and hang in spermaceti-white clusters from her setae and on surrounding objects. In the course of a few hours they become ciliated and swim about freely. The brood-pouch in[480] Thecidium is median, in the convex lower shell, in Cistella it is paired, and arises by the pushing in of the lateral walls of the body in the region just behind the horse-shoe-shaped tentacular arms; the renal ducts, which also serve as oviducts, open into these lateral recesses.
In the female Thecidium (Fig. 317) the two median tentacles which lie just behind the mouth are enlarged and their ends somewhat swollen; they are bent back into the brood-pouch, and to them the numerous larvae are attached by a short filament inserted into the second of the four segments into which the larva is divided. In Cistella a similar filament attaches the larvae to the walls of the brood-sac; thus they are secured from being washed away by the currents constantly flowing through the mantle cavity of the mother.
Fig. 317.—Brood-pouch of Thecidium mediterraneum. (After Lacaze-Duthiers.) Part of the wall of the pouch has been removed to show the clusters of larvae.
In Cistella the larva consists at first of two segments, but the anterior one divides into two, so that in the free swimming larva we find three segments, the hindermost somewhat longer and narrower than the others and destined to form the stalk. About the time of the appearance of the second segment four red eye-spots arise in the anterior segment, which tends to become constricted off from the others, and may now be termed the head. It gradually becomes somewhat umbrella-shaped, develops cilia all over its surface and a special ring of large cilia round its edge.
In the meantime the second or mantle segment has grown down and enveloped the stalk, and four bundles of setae have[481] arisen from its edge. In this stage the larva leaves its mother’s shell and swims out into the world of water to look for a suitable place on which to settle down. This is the only stage in the life history of a Brachiopod when the animal is locomotor, and can serve to spread its species. The extreme minuteness of the larva and the short time it spends in this motile condition probably accounts for the fact that Brachiopods are extremely localised. Where they do occur they are found in great numbers, rocks being often almost covered with them, but they are not found over large areas. When viewed under a microscope the larvae seem to be moving with surprising rapidity, but judging from the analogy of other forms, it seems doubtful if they swim a yard in an hour.
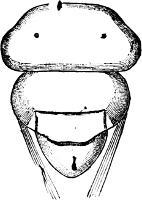
Fig. 318.—Young larva of Cistella neapolitana, showing three segments, two eye-spots, and two bundles of setae. (After Kowalevsky.)
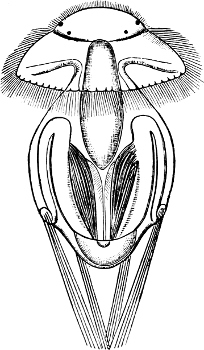
Fig. 319.—Full-grown larva of Cistella neapolitana, with umbrella-shaped head, ciliated. (After Kowalevsky.)
Frequently the larva stands on its head for some time, as if investigating the nature of the rocks on which it may settle; it is extremely contractile, turning its head from time to time, and seldom retaining the same outline for any length of time; the setae are protruded, and at times stick out in every direction; they are possibly defensive in function. When fully stretched out the larva is about ⅓ mm. long, but it frequently shortens its[482] body to two-thirds of this length. The larvae are of a pinkish red colour, with eye-spots of ruby red. Their colour renders them difficult to discern when they are swimming over the red coralline rocks upon which they frequently settle. After swimming about for a few hours the larva fixes itself finally, apparently adhering by some secretion produced by the stalk segment. The folds of the second or body segment then turn forward over the head, and now form the ventral and dorsal mantle folds; these at once begin to secrete the shell on their outer surfaces. The head with its eye-spots must be to some extent absorbed, but what goes on within the mantle is not accurately known. The setae drop off and the tentacular arms begin to appear as a thickening on the dorsal lobe of the mantle. They are at first circular in outline. The various changes which the larva passes through are well illustrated by Morse for Terebratulina, which spawns at Eastport, Me., from April till August. The different stages are represented in outline in Fig. 320, taken from his paper.
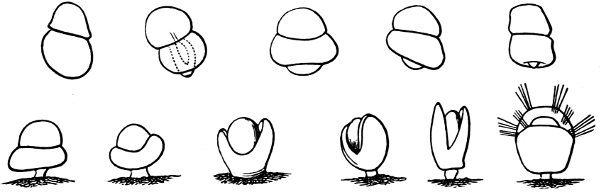
Fig. 320.—Stages in the development of the larva of Terebratulina septentrionalis. (After Morse.) The youngest larva has two segments, a third then appears, the larva then fixes itself, and the second segment folds over the first and develops bristles round its edge.
There is little to be said about the habits and natural history of the Brachiopoda. When once the larva has settled down, the animal never moves from the spot selected; occasionally it may rotate slightly from side to side on its stalk, and from time to time it opens its shell. As so frequently is the case with sessile animals, the sense organs are reduced to a minimum, the eyes of the larva disappear, and the only communication which the[483] animal has with the world around it is by means of the currents set up by the cilia on the tentacles.
In spite of the absence of any definite eyes, Thecidium, according to Lacaze-Duthiers, is sensitive to light; he noticed for instance that, when his shadow fell across a number of these animals he was watching in a vessel, their shells, which had been previously gaping, shut up at once.
In Cistella the tentacles can be protruded from the open shell, and in Rhynchonella the spirally-coiled arms can be unrolled and extruded from the shell, but this does not seem to have been observed in other genera, with the possible exception of Lingula. The food of these animals consists of minute fragments of animal and vegetable matter, a very large proportion of it being diatoms and other small algae.
Fig. 321.—Figures illustrating the tubes in which Lingula anatifera lives. The upper figure is a view of the trilobed opening of the tube. The lower figure shows the tube in the sand laid open and the animal exposed. The dotted line indicates the position of the body when retracted. The darker portion is the tube of sand agglutinated by the secretion of the stalk. (After François.)
Lingula differs markedly from the other members of the group, inasmuch as it is not firmly fixed to a rock or some such body by a stalk or by one of its valves, but lives in a tube in the sand. Some recent observations of Mons. P. François[427] on living specimens of Lingula anatifera which he found living in great numbers on the sea-shore at Nouméa in New Caledonia may be mentioned. The presence of the animal is shown by a number of elongated trilobed orifices which lead into the tube in which the Lingula lives. The animals, like most other Brachiopods, live well in captivity, and he was able to watch their habits in the aquaria[484] of his laboratory. The Lingula place themselves vertically; the anterior end of the body just reaches the level of the sand; the three lobes into which the orifice of the tube is divided corresponding with the three brushes of setae which project from the anterior rim of the mantles. These setae are described by Morse as projecting in the form of three funnels; currents of water are seen continually passing in at the side orifices and out through the central. The tube consists of two portions: an upper part, which is flattened to correspond with the flat shape of the body, and a lower part, in which the stalk lies. The upper part is lined with a layer of mucus, but the sand is not glued together to form a definite tube. The lower part of the stalk, or the whole when the animal is contracted, is lodged in a definite tube composed of grains of sand agglutinated by mucus, probably secreted from the walls of the stalk. At the least sign of danger the stalk is contracted violently, and the body is withdrawn to the bottom of the upper portion of the tube. The rapid retreat of the animal is followed by the collapse of the sand at the mouth of the tube, and all trace of the presence of the Lingula is lost.
The shells of this species are frequently rotated through a small angle upon one another, a movement which is prevented in the Testicardines by the hinge. In very young transparent specimens François was able to observe the movements of the fluid in the system of tubules which penetrate the mantle; these tubules are figured by him, and Fig. 315 is taken from his illustration.
Davidson in his Monograph on the British Fossil Brachiopoda states that the largest “recent Brachiopod which has come under my notice is a specimen of Waldheimia venosa Solander, measuring 3 inches 2 lines in length, by 2 inches in breadth, and 1 inch 11 lines in depth.” It was found in the outer harbour of Fort William, Falkland Islands, in 1843. A specimen of Terebratula grandis from the Tertiary deposits, however, exceeds this in all its dimensions. Its length was 4½ inches, its breadth 3 inches 2 lines, and its depth 2 inches 2 lines.
Brachiopods are very localised; they live in but few places,[485] but when they are found they usually occur in great numbers. During the cruise of the Challenger, dredging was conducted at 361 stations; at only 38 or 39 of these were Brachiopoda brought up. Mr. Cuming, quoted by Davidson, records that after a great storm in the year 1836, he collected as many as 20 bushels of Lingula anatifera on the sea-shore at Manilla, where, he relates, they are used as an article of food. It has been suggested above that their abundance in certain localities is due to their limited powers of locomotion, which are effective but for a few hours, the larva being, moreover, so minute that unless borne by a current it could not travel far from its parent. When once settled down it has little to fear from the attacks of other animals. The size of its shell relative to its body would deter most animals from regarding it as a desirable article of food, and as far as is known at present the Brachiopoda suffer but little from internal parasites, the only case I know being a minute parasitic Copepod belonging to a new and as yet unnamed genus which I found within the mantle cavity of Cistella (Argiope) neapolitana in Naples. Their slight value as an article of diet has doubtless helped to preserve them through the long periods of geological time, through which they have existed apparently unchanged.
Two of the recent genera of the family Lingulidae, Lingula and Glottidia, are usually found between tide-marks or in shallow water not exceeding 17 fathoms. Discina is also found about the low-tide level, but one species at any rate, Discinisca atlantica, has been dredged, according to Davidson, “at depths ranging from 690 to nearly 2425 fathoms.” Their larvae frequently settle on the shells of their parents, and thus numbers of overlapping shells are found clustered together. Crania is usually dredged from moderate depths down to 808 fathoms, adhering to rocks, lumps of coral, stones, and shells.
Of the Testicardines, Terebratula Wyvillei has probably been found at the greatest depth, i.e. 2900 fathoms, in the North Pacific. It is interesting to note that its shell is glassy and extremely thin. The Brachiopoda are, however, as a rule, found in shallower water; they abound up to a depth of 500 or 600 fathoms, after which they rapidly diminish with increasing depth. About one-half the named species occur at a depth of less than 100 fathoms.
[486]
The vertical range of depth of certain species is great; Terebratula vitrea is recorded from 5 to 1456 fathoms, T. Wyvillei from 1035 to 2900 fathoms. This is to some extent explicable since, after a certain depth has been reached, many of the external conditions, such as absence of temperature and light, must remain constant even to the greatest depths of the ocean.
The area of the ocean explored by dredging forms such an infinitesimal fraction of the whole, that it seems superfluous to consider the horizontal distribution of Brachiopods. A few facts may, however, be mentioned. Certain species, as Terebratula vitrea, T. caput serpentis, Waldheimia cranium, Megerlia truncata, and Discinisca atlantica, have a very wide if not cosmopolitan distribution. The second of the above named extends as far north as Spitzbergen, and as far south as Kerguelen Island. Many species are, on the other hand, very localised, and have hitherto only been found in one place. A very considerable number of these have been dredged off Japan and Korea, and this region may be to some extent regarded as the headquarters of the group.
The following species have been obtained within the limits of the British Area, as defined by Canon Norman, who has been good enough to revise the list, which is founded on that drawn up by Davidson in his Challenger Report. Their range of bathymetric distribution is given in the column on the left.
| Depth in Fathoms |
|||
| 0 to | 1180. | Terebratulina caput serpentis Lin. | Oban, and off Cumbrae Islands, Loch Torridon, Scotland, off Belfast |
| 8 to | 25. | Terebratula (Gwynia) capsula Jeff. | Belfast Bay, E. and S. coast of Ireland, Plymouth, Weymouth, and Guernsey |
| 5 to | 690. | Waldheimia cranium Müller | North British seas. Off Shetland |
| 75 to | 725. | Waldheimia septigera Lovén | North British seas. Off Shetland |
| 20 to | 600. | Terebratella spitzbergenensis Dav. | N.N.W. of Unst, Shetland |
| 18 to | 364. | Argiope decollata Chemnitz | Two miles east of Guernsey |
| 20 to | 45. | Cistella cistellula S. Wood | Shetland, near Weymouth, S. coast of England |
| 650 to | 1750. | Atretia gnomon Jeff. | W. of Donegal Bay in 1443 faths. Between Ireland and Rockall, in 1350 faths.[487] |
| 10 to | 690. | Rhynchonella psittacea Gmelin. | Shetland and near Dogger Bank.This species is possibly fossil as well as recent |
| 3 to | 808. | Crania anomala Müller | Loch Fyne, North of Scotland |
| 690 to | 2425. | Discinisca atlantica King | W. of Donegal Bay in 1366 faths., W. of Ireland in 1240 faths., off Dingwall Bay |
The table of classification here appended is that suggested by Mr. Davidson in his Monograph on the Recent Brachiopoda.
| I. TESTICARDINES | |
|---|---|
| Family | |
| A. Terebratulidae. | This includes the majority of genera and of species, the latter, without counting uncertain species, amounting to sixty-eight. Examples: Terebratula, Terebratella, Terebratulina, Waldheimia, Megerlia, Argiope, Cistella. |
| B. Thecidiidae. | This family contains one genus, Thecidium, with two species. |
| C. Rhynchonellidae. | This family is made up of eight species, six of which belong to the genus Rhynchonella, and two to Atretia. |
| II. ECARDINES | |
| D. Craniidae. | This family comprises the four species of Crania. |
| E. Discinidae. | This family contains one species of Discina and six of Discinisca. |
| F. Lingulidae. | This family consists of eight species of Lingula and three of Glottidia. |
It is impossible to come to any satisfactory conclusion as to the position of the group Brachiopoda with relation to the rest of the animal kingdom. They have, in accordance with the views of various investigators, been placed in close connexion with many of the large groups into which the Invertebrates are split up. The Mollusca, the Tunicata, the Polyzoa, the Chaetopoda, the Gephyrea, and of recent times such isolated forms as Phoronis and Sagitta, have all in turn had their claims advanced of relationship to this most ancient group. As far as I am in a position to judge, their affinities seem to be perhaps more closely with the Gephyrea and with Phoronis than with any of the other[488] claimants; but I think even these are too remote to justify any system of classification which would bring them together under a common name. Investigation into the details of the embryology of the group, more especially into that of the Ecardines, might throw some light on this subject, and it is much to be desired that this should be undertaken without delay. That the group is a most ancient one, extending from the oldest geological formations, we know, that the existing members of it have changed but little during the vast lapse of time since their earliest fossil ancestors flourished, we believe; but we are in almost total ignorance of the origin or affinities of the group, and we can hardly hope for any light on the subject except through embryological research.
[489]
PART II
PALAEONTOLOGY OF THE BRACHIOPODA
BY
F. R. COWPER REED, B.A., F.G.S.
Trinity College, Cambridge
[491]
INTRODUCTION—DIVISION I. ECARDINES—EXTERNAL CHARACTERS—INTERNAL CHARACTERS—DIVISION II. TESTICARDINES—EXTERNAL CHARACTERS—INTERNAL CHARACTERS—SYNOPSIS OF FAMILIES—STRATIGRAPHICAL DISTRIBUTION—PHYLOGENY AND ONTOGENY
The wide distribution and vast abundance of the Brachiopoda throughout the whole series of geological formations make this group of especial importance to the student of the past history of the earth; and the zoologist must always regard the fossil forms with peculiar interest, because they not only largely outnumber the living representatives, but comprise numerous extinct genera, and even families, exhibiting types of structure and characters entirely absent in the modern members of the group. It is a most fortunate circumstance that the excellent state of preservation in which we frequently find them, and the immense amount of material at our disposal, enable us to determine with accuracy and certainty the internal characters of the shells in the great majority of cases. But it is only since the beginning of the present century that our knowledge of the anatomy of the soft parts of the living animal has rendered any tracing of homologies possible. In the case of features in fossil extinct types the interpretation must be to some extent doubtful. Barrande, Clarke, Davidson, Hall, King, Oehlert, Waagen, de Verneuil, and a host of other workers have contributed to the information which we now possess; and their works must be consulted for details of the subject.[428]
[492]
Since all Brachiopods are inhabitants of the sea, the geologist at once recognises as a marine deposit any bed which contains their remains. Under favourable conditions they swarmed in the seas of Palaeozoic and Mesozoic times. Beds of limestone are frequently almost entirely composed of their shells, as, for instance, some of the Devonian limestones of Bohemia. Often they give the facies to the fauna and outnumber in species and individuals all the other organisms of the period. The Ungulite Sandstone (Cambrian) of Russia and the Productus Limestone of the Salt Range in India of Carboniferous and Permian age are well-known examples.
Many species seem to have been gregarious in habit; thus Productus giganteus of the Carboniferous Limestone may generally be found in crowded masses, as in some localities in Yorkshire.
The fact that certain species of Brachiopods characterise definite stratigraphical horizons or “zones” gives them occasionally an importance equal to that of Graptolites; for instance, the Ecardinate species Trematis corona marks a set of beds in the Ordovician, and the isolated Stringocephalus Burtini is restricted to the upper part of the Middle Devonian, giving to the limestone on that horizon its distinctive name. It is noteworthy also how certain species affect a sandy and others a calcareous sea-bottom, so that beds of the same age show differences in their Brachiopod fauna owing to a dissimilar lithological composition.
While few of the recent Brachiopods reach a large size, some of the extinct species measure several inches in breadth, but the great Productus giganteus attained the width of even a foot.
The bright colours of the shells of the living animals are not generally preserved amongst the fossil species from the older rocks; yet in a Carboniferous Terebratula we can even now detect the purple bands in some specimens, and a Cretaceous Rhynchonella similarly exhibits its original colour.
The Brachiopoda are evidently a group in its decline, as the geological record shows; but they date back from the earliest known fossiliferous rocks, in which the Ecardinate division is alone represented. As we ascend through the stratigraphical series the number and variety of genera and species belonging to[493] both divisions rapidly increase until in the united Ordovician and Silurian there are nearly 2000 species and about 70 genera. From this point of maximum development down to the present day there is a gradual decrease in numbers.
According to Davidson, at least 17 Upper Tertiary species are still living on our sea-bottoms; and many recent Mediterranean forms occur in the Pliocene rocks of the islands and shores of that sea, and in the Crags of East Anglia.
A brief review of the chief characteristics of fossil Brachiopoda is given below. Those genera which have the greatest zoological or geological importance can alone be noticed owing to the exigencies of space.
A considerable diversity of external form is met with even in this division, from the limpet-like Discina to the flattened tongue-shaped Lingula. The valves have most commonly a smooth external surface with delicate growth-lines; but sometimes pittings (Trematis) or radiating ribs (Crania) are present, and in a few forms the shell is furnished with spines (Siphonotreta), which perhaps serve to anchor it in the soft mud of the sea-bottom. The usual mode of fixation was by means of the pedicle (= peduncle or stalk), which either (1) passed out simply between the posterior gaping portion of the valves (Lingula), or (2) lay in a slit in the ventral valve (Lingulella), or (3) pierced the substance of the latter valve by a definite foramen (Discina). The first-mentioned condition of the pedicle seems the most primitive. Rarely the pedicle was absent, and the shell was attached by the whole surface of the ventral valve (Crania, p. 467).
The two valves in the fossil Ecardines were held together by muscular action, though in some families (Trimerellidae) we see traces of articulating processes. The “hinge line,” or line along which the valves worked as on a hinge, is in most forms more or less curved. A “hinge area” (i.e. that portion of the shell generally smoother than other parts of the valves, more or less triangular in form, and lying between the beaks on one or both sides of the hinge line), is usually absent in the Ecardines.
[494]
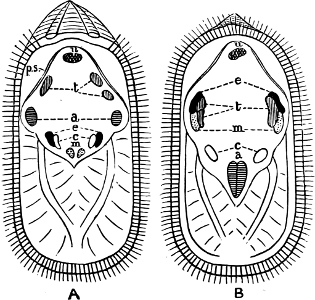
Fig. 322.—Muscle-scars of Lingula anatina. Inner surface of A, Pedicle-valve or ventral valve. B, Brachial or dorsal valve; p.s, parietal scar; u, umbonal muscle; t, transmedians; c, centrals; a.m.e, laterals (a, anteriors; m, middles; e, externals).
Fig. 323.—Trimerella. (After Davidson and King.) A, Inner surface of pedicle-valve or ventral valve: a, pseudo-deltidium; b, deltidial slope; c, deltidial ridges; d, areal borders; e, cardinal callosities; f, cardinal facet; g, lozenge; i, umbonal chambers separated by cardinal buttress; j, platform; k, platform vaults; l, median plate; m, median scars; n, anterior scars; o, lateral scars; p, post-median scars; q, crown crescent; r, side or lateral crescent; s, end or terminal crescent; t, transverse scars; u, archlet (vascular sinuses); w, sub-cardinal scars; x, umbo-lateral scars. B, Brachial or dorsal valve: e, cardinal sockets; j, platform; k, platform vaults; l, median plate; m, median scars; n, anterior scars; q, crown crescent; r, side or lateral crescent; s, end or terminal crescent; t, transverse scars; u, archlet (vascular sinuses); v, cardinal scars; w, sub-cardinal scars.
Owing to the rarity of well-preserved interiors of valves in this division, our knowledge of their internal characters is still far from satisfactory. The arrangement of the muscular impressions varies greatly amongst extinct genera, but we are often able to interpret them with a considerable amount of certainty by a study of the scars and the muscles of the well-known recent Lingula (Fig. 322). The extreme specialisation of the muscles in many of the earliest genera (e.g. Lingula) is remarkable, and points to a long but so far undiscovered ancestry in pre-Cambrian times.[429] In fossil species of Crania and Lingula the muscle-scars correspond closely with those in the living representatives of these genera. In the most highly specialised family of the Ecardines—the Trimerellidae—we meet with features of peculiar interest.[430] The muscle-scars in this family (Fig. 323, A, B) are most remarkable for the development of the so-called “crescent,” (q.r.s.) which skirts the posterior margin of both valves as a sub-cardinal impression. It is believed to be the trace of a strong post-parietal muscular wall, analogous in position to that of Lingula. The three pairs of “lateral” muscle-scars in the latter genus seem to be represented by the “terminal” (s) and “lateral” (r) scars on the crescent[495] of the Trimerellidae. A pair of “transverse” scars (t) occurs in each valve between the “terminals” and the antero-lateral edge of the “platform” (j). “Cardinal” (v), “sub-cardinal” (w), and “umbo-lateral” (x) scars also occur. The median impression which covers the “platform” (j) consists of a central, lateral, and usually an anterior pair of scars; and the impressions of the genital organs, according to Davidson and King, lie medianly posterior to the “platform.” The “platform” itself is a more or less conspicuous central calcareous elevated area occurring in each valve, but most developed in the dorsal; in some cases it is double-chambered with tubular cavities (“platform vaults,” Fig. 323, A, B, k), in others it is more or less solid. It appears to have originated through a posterior shifting of the central muscular bands, that they might be inserted behind the liver; at the same time a deposition of shelly material, to form fulcra to work the heavy valves, took place at these points. The tunnelling-out of the platform was probably due[496] to the continual pressure of the lobes of the liver. The division of the umbonal cavity into definite chambers in Monomerella, and to a less extent in other members of this family, appears, according to Davidson and King, to have been caused by pressure of the ovarian lobes.
In connexion with the foregoing remarks on the development of the “platform,” it may be mentioned that the paths along which the muscle-bands move, as the shell of Brachiopods increases in size, are marked by elongated scars, and often by shelly deposits; and when the members of a muscle-pair come into juxtaposition these shelly deposits (which act as fulcra for the muscles) combine, and by the growth of the shell form a septum, as in the case of the median septum of Lingulepis.
The Obolidae show some important features in the internal impressions. Obolella crassa (Hall) may be taken as a well-known type of the family. In this species a pair of small scars, one on each side of the pedicle-groove, lies close under the hinge line in the ventral valve. There is also a well-marked scar for the insertion of the pedicle-muscle at the end of the pedicle-groove. A pair of much elongated lateral impressions extending forward from the “cardinals” may be homologous with the “laterals” of Lingula; and the two small central scars between them may be compared with the “centrals” of Lingula which are in a somewhat similar position. In the dorsal valve of O. crassa a pair of “cardinals” is found, and on each side of a low median rounded ridge are two small “central” scars. Indistinct “lateral” scars arise close to or in the central area, and diverge anteriorly.
Sometimes a great concentration of muscle-scars occurs round the foramen in the ventral valve, as in Siphonotreta.
As regards the minute structure and composition of the shell in the Ecardines, we find that the Lingulidae and Discinidae have their shell composed of alternating layers of phosphate of lime and a corneous substance; the former layers are pierced by microscopic canals. The Craniidae have calcareous shells traversed by tubules, which divide into many fine branches near the external surface; a thin periostracum covers the exterior. The Trimerellidae have heavy thick calcareous shells, for which they required the previously-described elaborate arrangement of muscles to open and shut them.
[497]
It is to this division that the great majority of the Brachiopoda belong; and the diversity of form, of ornamentation, and of internal characters is correspondingly greater than in the Ecardines.
A transversely or longitudinally oval shape of shell is the commonest; but sometimes it is triangular, as in Rhynchonella (Fig. 327), or bilobed, as in Pygope (= Terebratula diphya). The ventral valve is usually more convex than the dorsal, and the former may be prolonged into a tube by the accelerated growth and infolding of the anterior and lateral margins, producing a very abnormal form (Proboscidella). The external surface of the valves is frequently ornamented with more or less prominent radiating ribs; and fine concentric growth-lines are commonly shown, and may be developed into coarse ridges or wrinkles, particularly in old individuals. The members of the family Productidae are usually furnished with tubular spines, which are sometimes of great length, and served to anchor the free shells in the mud, or were twisted round Crinoid stems and similar objects.
In the ventral valve of many genera there is a median sinus, with a corresponding fold in the dorsal valve, and rarely vice versâ; sometimes the fold and sinus are double.
The hinge line is either curved or straight, and the valves are articulated by means of a pair of “hinge-teeth” (Fig. 329, t) in the ventral valve, which fit into corresponding sockets in the opposite valve. Some genera have the teeth very rudimentary, or have lost them altogether. The teeth are frequently supported by “dental plates,” and the sockets by “socket plates” (e.g. Conchidium, Figs. 324, 325). A few genera with a long hinge line have the whole of it denticulated (Stropheodonta). In the dorsal valve medianly close under the hinge line is a shelly protuberance—the “cardinal process”—to which the diductor muscles are attached. It is sometimes of great length and forked (Stringocephalus, Fig. 326), or tripartite, or even quadripartite; but in Rhynchonella and some other genera it is rudimentary.
[498]
Fig. 324.—Conchidium galeatum. Wenlock Limestone.
Fig. 325.—Conchidium galeatum. Transverse section. d, Dorsal valve; d.s, dorsal septum; s, socket plate; v, ventral valve; v.s, ventral septum; d.p, dental plate.
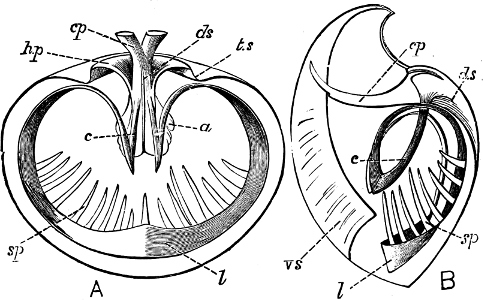
Fig. 326.—Stringocephalus Burtini. (Modified from Woodward.) Devonian. A, Interior of dorsal valve. B, Side view of interior of shell; a, adductor (= occlusor) scars; c, crura; c.p, cardinal process; d.s, dorsal septum; h.p, hinge plate; l, brachial loop; s.p, shelly processes; t.s, dental sockets; v.s, ventral septum.
A “hinge area” (Fig. 334, c.a) is often present on one or both valves, and may be of great size, as in Clitambonites, but in Productus it is wholly absent. In those genera that possess it a triangular fissure—the “deltidial fissure”—frequently traverses it on both valves; in the dorsal valve the fissure is merely the space between the dental sockets, and may be occupied by the cardinal- process (Fig. 334, C) or covered by a shelly plate—the “chilidium.” In the ventral valve it gives passage to the pedicle, and may be partly or entirely closed by a similar plate (Fig. 334, d) known as the “pseudo-deltidium,” especially large in Clitambonites, or remain open (Orthis). This pseudo-deltidium is a primitive character, and arises in an early stage of the[499] development as a shell-growth on the dorsal side of the animal, becoming attached to the ventral valve subsequently. The pedicle in many genera passes out through a special foramen in the beak of the ventral valve; and its proximal portion is often embraced by a pair of small plates—the deltidial plates or “deltidium”—which are formed on lateral extensions of the ventral mantle lobe, according to Beecher. These plates lie on each side of the pedicle, or grow round and unite in front of it (Rhynchonella, Fig. 327), or constitute merely its anterior border (Terebratula, Fig. 328). In some cases this foramen becomes closed in old age.
Fig. 327.—Rhynchonella Boueti. (Cornbrash.) d, Deltidium; f, foramen.
Fig. 328.—Terebratula sella. (Lower Greensand.) d, Deltidium; f, foramen.
The dorsal valve in a few cases has its beak perforated by a foramen—the “visceral foramen.” This foramen is in no way connected with the pedicle foramen, but points perhaps to the existence in the early Testicardinate genera of an anal aperture. In Athyris concentrica (Devonian) this foramen is connected internally with a cylindrical tube, which extends longitudinally to about one-third the length of the valve. In Centronella the aperture in the cardinal plate is rounded and complete; and in Strophomena and its allies the opening lies between the cardinal processes. If this feature is correctly interpreted, it suggests a retrogression of the group since Palaeozoic times not only in numbers, but in structure; and other evidence points the same way.
The interior of the shell is sometimes more or less divided up by septa. A median septum occurs in one or both valves of many genera as a low ridge or strongly developed partition (Waldheimia,[500] Fig. 329, ss; and Stringocephalus, Fig. 326, B, v.s). Conchidium (Fig. 325) has its dental plates of great size, and uniting to form a V-shaped chamber or “spondylium,” supported by a median double septum; and by means of these with a pair of septa and the large socket-plates in the dorsal valve the interior of the shell of this genus is divided up into several chambers.
The interiors of several other genera are somewhat similarly divided up.
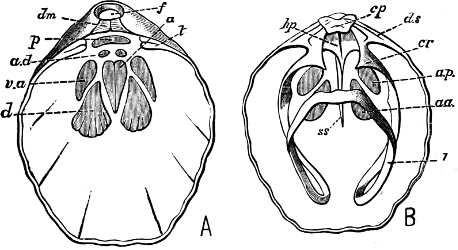
Fig. 329.—Waldheimia (Magellania) flavescens. A, Interior of ventral valve: a, adductor scars; v.a, ventral adjustors; d, divaricators; a.d, accessory divaricators; p, peduncular muscle; dm, deltidium; f, foramen; t, teeth. B, Interior of dorsal valve: a.a, anterior adductor (occlusor) scars; a.p, posterior adductor (occlusor) scars; c.p, cardinal process; cr, crura; d.s, dental sockets; hp, hinge-plate; l, brachial loop; ss, septum. (After Davidson.)
In the Carboniferous genus Syringothyris two special plates, situated between the dental plates, are rolled into an incomplete tube, so as to enclose probably the anal extremity of the alimentary canal; and in several genera a sub-umbonal “cardinal plate” is present, which is perforated (Athyris) or slit in some cases for the passage of the anal tube.
For the support of the fleshy “spiral arms” the calcareous structures forming the “brachial apparatus” are of two main types—(1) the loop type; (2) the spiral-cone type. In the Strophomenidae no special calcareous support seems to have been usually present (Fig. 334), though in some species of Leptaena spirally-grooved elevated areas supported the fleshy arms; in the Productidae it is probable that the ridges enclosing the “reniform impressions” (Fig. 333, i) served for a similar purpose.
The Terebratulidae show the “loop type” of brachial apparatus. In Waldheimia (Fig. 329), which may be taken as an[501] example, we notice first in the dorsal valve the “crura” (cr), from which arise the two “descending branches” which run forwards and then are bent back to form the “ascending branches” which are united by the “transverse band.” In some genera the “ascending branches” may be reduced to mere points, and the “transverse band” become a median vertical plate; the “crura,” too, may be fused so as to form a “crural band”; and the “descending branches” may be connected by a cross band—the “jugal band.” In Stringocephalus (Fig. 326, l, s.p) the loop is furnished on its inner edge with radiating processes; and in Argiope the loop is simple, not reflected, and fused with marginal septa; while in the Thecidiidae it is more or less fused with the shell itself, and with the mass of calcareous spicules secreted by the mantle.
The “spiral-cone type” of brachial apparatus is found in the Spiriferidae, Atrypidae, and Koninckinidae, and consists of two spirally-enrolled calcified lamellae, forming two cones with their apices directed laterally (Spirifera, Fig. 330), or towards the interior of the dorsal valve (Atrypa, Fig. 332), or towards each other (Glassia); or forming two flat spirals in the same plane (Koninckinidae). A “jugal band” is generally present, but varies much in position, and in some genera has complicated posterior processes.
The Rhynchonellidae have no loop or spiral cones, but merely a pair of short “crura.”
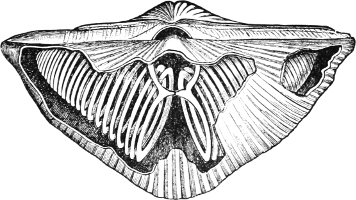
Fig. 330.—Spirifera striata. (Carboniferous Limestone.) Showing brachial spires.
The principal modifications in the attachments of the muscles in the Testicardines are illustrated by Productus giganteus (Fig. 333), Leptaena rhomboidalis (Fig. 334), and Waldheimia flavescens (Fig. 329).
In Productus (Fig. 333) we see in the ventral valve a pair of dendritic occlusor, often called adductor, impressions and a pair of large flabellate divaricator impressions. In the dorsal valve the large “cardinal process” served for the attachment of the divaricator, and a low median septum separated the dendritic[502] occlusor scars, which are rarely divisible into anterior and posterior pairs.
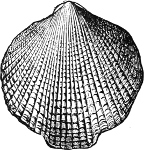
Fig. 331.—Atrypa reticularis. (Wenlock Limestone.)
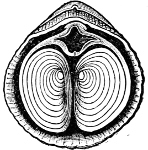
Fig. 332.—Interior of the same, seen from the dorsal side, showing brachial spires. (After Hall.)
In Leptaena (Fig. 334) the occlusor scars (a) in the ventral valve are narrow and median, and are enclosed by a pair of flabelliform divaricator impressions (d.v); in the dorsal valve two pairs of occlusor scars (a.a, p.a) are well marked, and accessory posterior occlusor scars are traceable in some specimens. The vascular sinuses (v.s) and genital areas are conspicuous in many species of this and other genera.
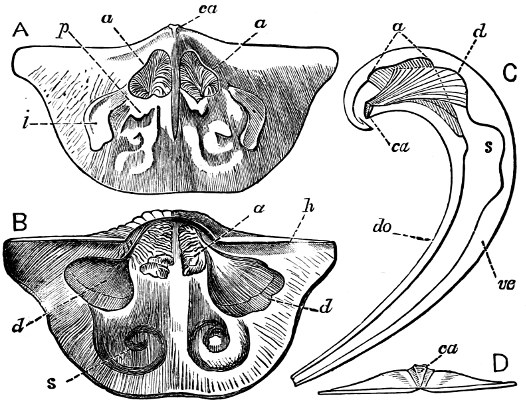
Fig. 333.—Productus giganteus. (After Woodward.) Carboniferous Limestone. A, Interior of dorsal valve. B, Interior of ventral valve. C, Transverse section of valves. D, Hinge line of A: a, occlusor scars; d, divaricator scars; i, “reniform impressions”; ca, cardinal process; h, hinge line; p, brachial prominence; s, cavity for spiral arms; do, dorsal valve; ve, ventral valve.
In Waldheimia (Fig. 329) a sub-umbonal “peduncular muscle”[503] scar (p) in the ventral valve has before it a pair of “accessory divaricator” scars (a.d) flanked by a pair of “ventral adjustor” (v.a) and a pair of “divaricator” impressions (d), between which lie the two occlusor scars (a). In the dorsal valve anterior and posterior pairs of occlusor scars (a.a, a.p) are visible.
The minute structure of the calcareous shell of the Testicardines is of flattened fibrous prisms inclined at a very acute angle to the surfaces. In many forms minute tubes more or less closely arranged pierce through the fibrous shell-substance; but in some genera (Productus) they do not reach the outer surface (see p. 468). Allied genera, however, differ much in the punctate or impunctate character of the shell.
Fig. 334.—Leptaena rhomboidalis. (Silurian.) A, External view of ventral valve. B, Interior of ventral valve: a, occlusor scars; d, pseudo-deltidium; d.v, divaricator scars; c.a, hinge area; t, teeth. C, Interior of dorsal valve: a.a, anterior occlusor scars; p.a, posterior occlusor scars; c.a, hinge area; c.p, cardinal process; d, chilidium; s, dental sockets; v.s, vascular sinuses.
Synopsis of Families
Shell elongated, composed of alternating chitinous and calcareous layers, the latter of which are perforated. Attached by a pedicle passing between apices of valves.
Arms have no calcified supports.
(For muscles see Fig. 322.)
Range.—Lower Cambrian to Recent.
Principal Genera.—Lingula, Lingulella, Lingulepis.
[504]
Shell varies in shape. Ventral valve provided with pedicular groove or foramen. Cardinal border thickened. No brachial supports. Shell composed of alternating chitinous and calcareous layers.
(For muscles see p. 496.)
Range.—Lower Cambrian to Devonian.
Principal Genera.—Obolus, Obolella, Kutorgina, Linnarssonia, Siphonotreta, Acrotreta, Neobolus.
Shell rounded, valves more or less conical, fixed by pedicle passing through slit or tubular foramen in ventral valve. No calcified brachial supports. Shell structure chitino-calcareous.
Range.—Ordovician to Recent.
Principal Genera.—Discina, Orbiculoidea, Trematis.
Shell calcareous, subcircular; fixed by surface of ventral valve; dorsal valve the larger, depressed-conical. Shell structure punctate.
Four principal muscular scars in each valve, with central triangular protuberance in ventral valve (see p. 476).
Range.—Ordovician to Recent.
Principal Genus.—Crania.
Shell thick, calcareous, inequivalve; beak of ventral valve usually prominent; rudimentary teeth maybe present; hinge area well developed, with pseudo-deltidium. In interior of valves muscular platform, “crescent,” and sometimes sub-umbonal chambers (see p. 494, Fig. 323).
Range.—Ordovician and Silurian; maximum in Wenlock.
Principal Genera.—Trimerella, Monomerella, Dinobolus, Rhinobolus.
Shell entirely free, or fixed by ventral valve or spines. Concavo-convex, more or less covered with tubular spines. Hinge line straight. Hinge-teeth absent or rudimentary.
Cardinal process prominent.
Reniform impressions in dorsal valve.
(For muscular impressions see p. 501, Fig. 333.)
Range.—Silurian to Permian. Genus Productus very characteristic of the Carboniferous.
Principal Genera.—Productus, Chonetes, Strophalosia, Proboscidella, Aulosteges.
[505]
Shell very variable in shape; concavo-convex, plano-convex, or biconvex; hinge line usually straight; frequently with an area on each valve; foramen may or may not be present. Shell structure near always punctate. Ventral valve usually furnished with hinge-teeth; and dorsal valve with cardinal process.
Brachial supports completely absent or very rudimentary.
(For muscular impressions see p. 502, Fig. 334.)
Range.—Wholly Palaeozoic.
Principal Genera.—Orthis, with many sub-genera, Clitambonites, Skenidium, Strophomena, Orthothetes, Leptaena, Stropheodonta, Plectambonites.
Shell plano-convex or concavo-convex. Brachial apparatus composed of two lamellae spirally enrolled in the same plane, or in the form of depressed cones, with the apices directed into the ventral valve.
Range.—Silurian to Lias.
Principal Genera.—Koninckina, Koninckella, Coelospira, Davidsonia.
Shell biconvex. Brachial apparatus consisting essentially of two descending calcareous lamellae which by spiral enrolment form a pair of laterally-directed cones (Fig. 330).
Range.—Chiefly Palaeozoic, but a few forms pass up into the Lias.
Principal Genera.—Spirifera, Cyrtia, Uncites, Athyris, Merista.
Brachial apparatus consists of two descending calcareous lamellae which bend outwards at the extremity of the crura and are coiled into two spiral cones, the apices of which either converge towards each other (Glassia) or towards the dorsal valve (Atrypa, Fig. 332), or diverge towards the dorsal valve (Dayia); shell structure impunctate.
Range.—Ordovician to Trias.
Principal Genera.—Atrypa, Dayia, Glassia.
Shell biconvex, hinge line usually curved.
Beak of ventral valve incurved, with foramen.
Calcareous brachial supports reduced to a pair of short curved crura.
The septa, dental and socket plates may be highly developed and divide up the cavity of the shell into chambers (Stenochisma, Conchidium).
Shell structure fibrous, rarely punctate; muscular impressions as in Terebratulidae.
Range.—Ordovician to Recent: majority of the genera are Palaeozoic.
Principal Genera.—Rhynchonella (Fig. 327), Stenochisma, Stricklandia, Conchidium.
[506]
Shell structure punctate.
Arms supported by a calcareous loop, usually bent back on itself.
(For muscular impressions see p. 502, Figs. 328, 329.)
Beak of ventral valve perforated by foramen, furnished with deltidium.
Range.—Devonian to Recent; maximum development in Mesozoic times.
Principal Genera.—Terebratula, Terebratulina, Waldheimia, Terebratella, Kingena, Magas, Centronella.
Large foramen for passage of pedicle. Marginal septa present in both valves. Calcareous brachial loop follows margin of shell and is more or less fused with the septa. Shell structure punctate.
Range.—Jurassic to Recent.
Principal Genera.—Argiope, Cistella.
Shell subcircular, punctate. Cardinal process highly developed, bifid. Brachial apparatus composed of two calcareous free lamellae, prolonged at first downwards, then bent back, upwards and outwards to run parallel to margin of shell and to unite in front, thus constituting a wide loop.
Range.—Silurian and Devonian.
Sole Genus.—Stringocephalus.
Shell usually fixed by beak of ventral valve, plano-convex. Sub-cardinal apophysis in ventral valve for attachment of occlusors. Marginal septa in dorsal valve. Calcareous brachial loop more or less fused with shell, and with calcareous spicules of mantle. Shell structure: inner layer fibrous, outer layer tubulated.
Range.—Carboniferous to Recent.
Principal Genera.—Thecidium, Oldhamina.
It is remarkable that some of the earliest types of Brachiopoda exist generically unchanged at the present day. Such are Lingula, ranging from the Cambrian; Discina and Crania, ranging from the Ordovician; and amongst the hinged forms Terebratula from the Devonian, and Rhynchonella from the Ordovician.
In the lowest Cambrian (Olenellus beds) the most important genera are Linnarssonia and Kutorgina. The hinged forms appear in the Cambrian, being represented by Orthis; but the majority in this formation belong to the Ecardines. Lingula, Lingulella, and Obolella are characteristic.
[507]
In the Ordovician many new genera of the Testicardines make their appearance, such as Strophomena, Leptaena, Atrypa, Rhynchonella, Clitambonites, etc., but the extraordinary abundance and variety of Orthis is most remarkable. The Ecardines are reinforced by such forms as Trematis and Siphonotreta. It is, however, in the Silurian that the Testicardinate Brachiopoda attain their maximum, for in addition to a great development of species amongst the older forms, a host of new genera for the first time occur here (Spirifera, Athyris, Conchidium, Stricklandia, Chonetes, Cyrtia, etc.); and the Trimerellidae are especially characteristic of the Wenlock.
With the commencement of Devonian times many species and genera become extinct, but new forms come in (Terebratula, Orthothetes, Productus, etc.), and some genera are wholly confined to this formation (Uncites, Stringocephalus). The Carboniferous is marked by the maximum development of Productus and Spirifera; Orthothetes, Stenochisma, and Athyris are also abundant, but there is a considerable extinction of the older genera and species, and a great diminution in the number of individuals and species of those that persist.
A further reduction occurs in the Permian, where the most important genera are Productus, Strophalosia, and Stenochisma; but Aulosteges is a new form peculiar to this period. In the Trias a new era commences; the principal families and genera of the older rocks disappear entirely; a few spire-bearing genera persist (Spiriferina, Athyris), and the genus Koninckina is restricted to this formation.
The enormous development of species of the Terebratulidae and Rhynchonellidae is the most noticeable feature in Jurassic times; and a few ancient types linger on into the Lias (Spiriferina, Suessia, a sub-genus of Spirifera); Koninckella here occurs.
The Cretaceous Brachiopoda are closely allied to the Jurassic; Magas and Lyra are peculiar to the period, and the Terebratulidae and Rhynchonellidae are very abundant, together with the Ecardinate genus Crania.
With the commencement of Tertiary times the Brachiopoda have lost their geological importance, and have dwindled down into an insignificant proportion of the whole Invertebrate fauna.
The distribution of the Brachiopoda in past time is shown in the following table:—
[508]
| Palaeozoic | Mesozoic | |||||||||||
| C | ||||||||||||
| a | ||||||||||||
| r | ||||||||||||
| O | b | C | ||||||||||
| r | o | r | ||||||||||
| C | d | S | D | n | J | e | T | |||||
| a | o | i | e | i | P | u | t | e | ||||
| m | v | l | v | f | e | r | a | r | R | |||
| b | i | u | o | e | r | T | a | c | t | e | ||
| r | c | r | n | r | m | r | s | e | i | c | ||
| i | i | i | i | o | i | i | s | o | a | e | ||
| a | a | a | a | u | a | a | i | u | r | n | ||
| ECARDINES | n | n | n | n | s | n | s | c | s | y | t | |
| Lingulidae | Lingula | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ |
| Lingulella | ___ | |||||||||||
| Obolidae | Obolus | ___ | ___ | |||||||||
| Obolella | ___ | ___ | ||||||||||
| Kutorgina | ___ | ___ | ||||||||||
| Linnarssonia | ___ | |||||||||||
| Trematis | ___ | ___ | ||||||||||
| Siphonotreta | ___ | ___ | ||||||||||
| Acrotreta | ___ | |||||||||||
| Discinidae | Discina | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | |
| Craniidae | Crania | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | |
| Trimerellidae | Trimerella | ___ | ||||||||||
| Dinobolus | ___ | |||||||||||
| TESTICARDINES | ||||||||||||
| Productidae | Productus | ___ | ___ | ___ | ||||||||
| Chonetes | ___ | ___ | ___ | |||||||||
| Strophalosia | ___ | ___ | ___ | |||||||||
| Strophomenidae | Orthis | ___ | ___ | ___ | ___ | ___ | ||||||
| Skenidium | ___ | ___ | ||||||||||
| Clitambonites | ___ | |||||||||||
| Strophomena | ___ | ___ | ||||||||||
| Stropheodonta | ___ | ___ | ___ | |||||||||
| Leptaena | ___ | ___ | ___ | ___ | ||||||||
| Orthothetes | ___ | ___ | ___ | |||||||||
| Davidsonia | ___ | |||||||||||
| Koninckinidae | Koninckina | ___ | ||||||||||
| Koninckella | ___ | |||||||||||
| Spiriferidae | Spirifera | ___ | ___ | ___ | ___ | |||||||
| Spiriferina | ___ | ___ | ___ | ___ | ___ | |||||||
| Cyrtia | ___ | ___ | ___ | |||||||||
| Syringothyris | ___ | |||||||||||
| Uncites | ___ | |||||||||||
| Athyris | ___ | ___ | ___ | ___ | ___ | |||||||
| Merista | ___ | ___ | ||||||||||
| Retzia | ___ | ___ | ___ | ___ | ___ | |||||||
| Atrypidae | Atrypa | ___ | ___ | ___ | ___ | ___ | ___ | |||||
| Dayia | ___ | |||||||||||
| Coelospira | ___ | |||||||||||
| Rhynchonellidae | Rhynchonella | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | |
| Stenochisma | ___ | ___ | ___ | |||||||||
| Stricklandia | ___ | |||||||||||
| Conchidium | ___ | ___ | ||||||||||
| Terebratulidae | Terebratula | ___ | ___ | ___ | ___ | ___ | ___ | ___ | ___ | |||
| Terebratulina | ___ | ___ | ___ | ___ | ||||||||
| Waldheimia | ___ | ___ | ___ | ___ | ||||||||
| Terebratella | ___ | ___ | ___ | ___ | ||||||||
| Kingena | ___ | ___ | ||||||||||
| Magas | ___ | |||||||||||
| Centronella | ___ | ___ | ___ | |||||||||
| Argiopidae | Argiope | ___ | ___ | ___ | ___ | |||||||
| Cistella | ___ | ___ | ___ | ___ | ||||||||
| Stringocephalidae | Stringocephalus | ___ | ||||||||||
| Thecidiidae | Thecidium | ___ | ___ | ___ | ___ | ___ | ||||||
| Oldhamina | ___ | |||||||||||
[509]
Wherever successive stages in the life history of an individual resemble in important anatomical features the adult individuals of other species occurring in successive members of a stratigraphical series, the development of the individual may be regarded as an epitome of the development of the species; it also generally throws light on the origin and relationships of allied genera and families.
In the case of the fossil Brachiopoda comparatively little work has yet been done in tracing their ontogeny or phylogeny, though the abundance, variety, and excellent state of preservation of the extinct species offer a promising field for investigation. It is to Dr. C. E. Beecher and other recent American palaeontologists that we owe our advance in this branch of the subject.
In the first place, in about forty genera, representing nearly all the leading families of the group, the important fact has been established of the presence of a common form of embryonic shell, termed the “protegulum,” which is “semicircular or semielliptical in shape with a straight or arcuate hinge line and no hinge area” (Beecher).[431] Its minute size and delicate texture cause its preservation to be rare, but its impression is not uncommonly left on the beak of the adult shell.
The main features of this embryonic shell are exhibited in the adult Lower Cambrian Brachiopod Obolus (Kutorgina) labradoricus (Billings); the sub-equal semielliptical valves have lines of growth running concentrically and parallel to the margin of the shell, and ending abruptly against the straight hinge line; and this indicates that there has been no change in the outline and proportions of the shell during its stages of growth, but only a general increase in size. It is very significant that we have here a mature type possessing the common embryonic characters of a host of widely separated genera, and we may therefore regard it as the most primitive form known.
Many genera pass through this so-called “Paterina” stage either in the case of both their valves, or more generally in the case of the dorsal valve only; but modifications in the form of the protegulum arise, which are due to the influence of[510] accelerated growth, by which features belonging to later stages become impressed on the early embryonic shell. The most variable and specialised valve—the ventral or pedicle valve naturally exhibits the effect of this influence first and to the greatest extent. The Palaeozoic adult forms of many species represent various pre-adult stages of the Mesozoic, Tertiary, and Recent species, as is especially well shown in the genera Orbiculoidea and Discinisca.
In the Strophomenoid shells the protegulum in the dorsal valve is usually normal, but in the ventral valve abbreviation of the hinge and curvature of the hinge line are produced by acceleration of the “Discinoid stage” in which a pedicle notch is present.
No marked variation has yet been noticed in the spire-bearing, or Terebratuloid, or Rhynchonelloid genera.
The form of the shell and the amount of difference in shape and size of the valves seem to be largely due to the length of the pedicle and its inclination to the axis of the body, as evidenced by the development of Terebratulina. A series showing progressive dissimilarity of the two valves arising from these causes can be traced from Lingula to Crania. The greater alteration that takes place in the ventral valve appears to be due to its position as lower and attached valve. If the pedicle is short a transversely-expanded shell with long hinge line results when the plane of the valves is vertical or ascending, but when the latter is horizontal a Discinoid form is found. This mode of attachment is often accompanied by a more or less plainly developed radial symmetry. Shells with long pedicles, on the other hand, are usually longer than wide.
The character of the pedicle-opening is of great significance from an evolutional and classificatory point of view, for the successive stages through which it passes in embryonic growth are chronologically paralleled by different genera, and are likewise accompanied by the successive acquisition of other important anatomical characters, as has been shown by Beecher and others. The first and simplest type of pedicle opening is in shells with a posterior gaping of the valves, where the pedicle protrudes freely between them in a line with the axis, and the opening is shared by both valves, though generally to a greater extent by the ventral valve. Paterina (= Obolus labradoricus) and Lingula furnish[511] examples of this type. In the second type the pedicle opening is restricted to the ventral valve, and the direction of the pedicle makes a right angle with the plane of the valves; in the lower forms the pedicle lies in a slit or sinus (Trematidae), but by further specialisation it becomes enclosed by shell growth so as to lie within the periphery, and finally becomes sub-central in some genera (Discinidae). The third type shows the pedicle opening confined to the ventral valve and sub-marginal. A pseudo-deltidium may preserve the original opening (Clitambonites); or this shelly plate may become worn away or reabsorbed in the adult so that the deltidial fissure through which the pedicle passes remains quite open (Orthidae). In the fourth type the incipient stage marks a return to the simple conditions of the first type; but ultimately a pair of deltidial plates develop, and may completely limit the pedicle opening below. Examples of this type are Spirifera and Rhynchonella. By means of these four types the Brachiopods have been divided into four Orders: the Atremata (type i.); the Neotremata (type ii.); the Protremata (type iii.); and the Telotremata (type iv.).
The Telotremata were the last to appear, but the four types of pedicle-opening with the various forms of calcareous brachial apparatus were in existence in the Bala period of the Ordovician.
As Paterina is the most primitive form of all, we may place it at the root of the phylogenetic tree. From it sprang the Atremata, which gave off the Neotremata and Protremata; the most primitive Neotremata seem to be the Trematidae, while the connecting link between the Protremata and Atremata is furnished by the Kutorginidae. From the genus Conchidium and its allies we may see how the Rhynchonellidae ushered in the Telotremata as an offshoot from the Protremata. The Telotremata subsequently gave off two main branches, which became specialised with the loop-bearing and spire-bearing forms respectively.
The evolution and mutual relationships of genera have been indicated with much probability by Hall, Clarke, and others. The Obolelloid type may be connected with the Linguloid by means of Lingulella and Linyulepis, while in Lingula itself we find the point of divergence for the ancestors of Trimerella, and for a line of variation culminating in Dignomia. The Palaeozoic Rhynchonelloids branched off at an early period from the same stock as Orthis, and are connecting links between this[512] genus and Mesozoic Rhynchonellae; and a whole series of genera exhibit intermediate stages of structure between the Rhynchonelloid and Pentameroid groups. The Terebratuloids can be traced back to the primitive type Renssoellaria; and amongst spire-bearing forms, the protean genus Spirifera can be split up into groups of species which diverge along lines tending to forms no longer congeneric. When we come to deal with specific differences we find frequently such a host of intermediate varieties that the separation of many species, as in the case of Mesozoic Terebratulae, is to a large extent arbitrary and artificial.
[513]
References to figures are printed in thick type (248, 197); to systematic position, in italics (391, 430)
END OF VOL. III.
MACMILLAN & CO.’S
PUBLICATIONS ON
NATURAL HISTORY, ETC.
ADLER (H).—Alternating Generations. A Biological Study of Oak Galls and Gall Flies. By Hermann Adler, M.D., Schleswig. Translated and edited by Charles R. Straton, F.R.C.S. Ed., F. E. S. $3.25.
BADENOCH (L. N.).—The Romance of the Insect World. By L. N. Badenoch. With Illustrations by Margaret J. D. Badenoch and others. 2d Edition. Gilt top. $1.25.
BALFOUR.—A Treatise on Comparative Embryology. By F. M. Balfour, M.A., F.R.S., Fellow and Lecturer of Trinity College, Cambridge. With Illustrations. Second Edition, reprinted without alteration from the First Edition. In 2 vols. 8vo. Vol. I., $4.50; Vol. II., $5.25.
BATESON (W.).—Materials for the Study of Variation, treated with especial Regard to Discontinuity in the Origin of Species. By William Bateson, M.A.. Fellow of St. John’s College, Cambridge. With many cuts. 8vo. $6.50.
BEDDARD (F. E.).—Animal Colouration. With 4 Coloured Plates, and Woodcuts in the Text. By Frank Evers Beddard, M.A., F.R.S., Prosector to the Zoölogical Society of London and Lecturer on Biology at Guy’s Hospital. 8vo. $3.50.
A Monograph, Structural and Systematic, of the Order Oligochæta. Large Post 8vo. Half cloth. 800 pp. Just ready.
CAMBRIDGE NATURAL HISTORY (The). Edited by J. W. Clark, M.A., S. F. Harmer, M.A., and A. E. Shipley, M.A. Medium 8vo.
This series, which will be complete in ten volumes, fully illustrated, and covering the Natural History of Invertebrate and Vertebrate Animals, is intended, in the first instance, for those who have not had any special scientific training, but an attempt will be made to combine popular treatment and popular language with the most modern results of scientific research.
The following volumes are likely to appear in the course of 1895:
Molluscs. By Rev. A. H. Cooke, M.A. Just ready.
Insects. By David Sharp, M.A., F.R.S. 2 vols.
Birds. By A. H. Evans, M.A.
CLAUS.—Elementary Text-Book of Zoölogy. By Dr. C. Claus. Translated and edited by Adam Sedgwick, M.A., with the assistance of F. G. Heathcote, B.A. Part I., General Part and Special Part: Protozoa to Insecta. Part II., Special Part: Mollusca to Man. With 706 Woodcuts. 2 vols. 8vo. $8.00.
ECKER.—The Anatomy of the Frog. By Alexander Ecker. Translated with numerous Annotations and Additions, by G. Haslam, M.D., and illustrated with 250 Wood Engravings and 11 Coloured Figures. 8vo. $5.25.
EIMER.—Organic Evolution as the Result of the Inheritance of Acquired Characters, according to the Laws of Organic Growth. By Dr. G. H. Theodor Eimer, Professor of Zoölogy and Comparative Anatomy in Tübingen. Translated by T. J. Cunningham, M.A., F.R.S.E., late Fellow of University College, Oxford. Part I., with 6 Figures in the Text. 8vo. $3.25.
FLOWER.—Mammals, Living and Extinct. By William Henry Flower, C.B., F.R.S., D.C.L., Director of the Natural History Departments, British Museum, and Richard Lydecker, B.A. 8vo. Cloth. Illustrated with 357 Figures. $6.00.
FOWLER (A. Warde).—Tales of the Birds. With Illustrations. New and cheaper Edition. $1.25.
A Year with the Birds. With Illustrations. $1.25.
GÜNTHER (Albert C. L. G.).—An Introduction to the Study of Fishes. With Index. Illustrated with 320 Wood Engravings. 8vo. $6.00.
HERTWIG (O.).—Text-book of the Embryology of Man and Mammals. By Dr. Oscar Hertwig. Translated from the Third German Edition by Edward L. Mark, Hersey Professor of Anatomy in Harvard University. With 330 Figures in the Text and two Lithographic Plates. 8vo. $5.25.
The Cell: its Anatomy and Physiology. By Dr. Oscar Hertwig, Professor in the University of Berlin. Translated by Henry Johnstone Campbell, M.D. With 168 Illustrations. In the Press.
HUXLEY and MARTIN.—A Course of Elementary Instruction in Practical Biology. By T. H. Huxley, LL.D., F.R.S., assisted by H. N. Martin, M.A., M.D., D.Sc., F.R.S. Revised Edition, Extended, and Edited by G. B. Howes and D. H. Scott. With a Preface by Professor Huxley. 12mo. $2.60.
KIRBY (W. F.).—Elementary Text-Book of Entomology. 2d Edition, Revised. With 87 Plates. 8vo. $3.00.
LANG.—Text-Book of Comparative Anatomy. By Dr. Arnold Lang, Professor of Zoölogy in the University of Zurich; Formerly Ritter Professor of Phylogeny in the University of Jena. With a Preface to the English Translation by Professor Dr. Ernst Haeckel, F.R.S., Director of the Zoölogical Institute in Jena. Translated into English by Henry M. Bernard, M.A. (Cantab.) and Matilda Bernard. Part I. Complete with Index and 383 Illustrations. 8vo. $5.50.
Professor Lang has here successfully carried out the very difficult task of selecting the most important results from the bewildering mass of new material afforded by the extensive researches of the last decades, and of combining them with great judgment. Besides this he has, more than any former writer, utilized the comparative history of development in explaining the structure of the animal body, and has endeavored always to give the phylogenetic significance of ontogenetic facts.—From Professor Haeckel’s Preface.
LUBBOCK (Sir J.).—On the Origin and Metamorphoses of Insects. With Illustrations. Nature Series. $1.00.
MIVART.—Lessons in Elementary Anatomy. By St. George Mivart, F.R.S., Lecturer in Comparative Anatomy at St. Mary’s Hospital. With upwards of 400 Illustrations. 16mo. $1.75.
PARKER.—A Course of Instruction in Zoötomy (Vertebrata). By T. Jeffery Parker. With seventy-four Illustrations. 12mo. $2.25.
WALLACE.—Works by Alfred Russel Wallace, LL.D., F.L.S. Darwinism. Being a Systematic Exposition of the Theory of Natural Selection, with Some of its Applications. With numerous Illustrations. $1.75.
“The present work contains the conclusions upon this great subject of thirty years of thought and observation.... A contribution of the first importance to the literature of the subject. At the same time it would be difficult to find a book more entertaining to the general reader. He writes with the sincerity and easy mastery which comes of fulness of knowledge. There can be no more interesting guide in that great wonderland of science in which he has been so long one of the chief discoverers.”—New York Times.
The Malay Archipelago; The Land of the Orang Utan and the Bird of Paradise. A Narrative of Travel. With Studies of Man and Nature. With Illustrations. Ninth Edition. 12mo. $1.75.
Contributions to the Theory of Natural Selection; and Tropical Nature and Other Essays. New edition. In one volume. 12mo. $1.75.
Island Life; or, The Phenomena and Causes of Insular Faunas and Floras. Including Revision and Attempted Solution of the Problem of Geological Climates. With Illustrations and Maps. New and cheaper Edition. 12mo. $1.75.
WIEDERSHEIM.—Elements of the Comparative Anatomy of Vertebrates. Adapted from the German of Robert Wiedersheim. By W. Newton Parker. With Additions. Illustrated with 270 Woodcuts. 8vo. $3.00.
WEISMANN.—Essays upon Heredity and Kindred Biological Problems. By Dr. August Weismann, Professor in the University of Freiburg in Breisgau. Edited by Edward B. Poulton, M.A., F.R.S., Selmar Schonland, and Arthur E. Shipley. 12mo. $2.00.
MACMILLAN & CO.,
66 FIFTH AVENUE, NEW YORK.
Columbia University Biological Series.
EDITED BY
HENRY FAIRFIELD OSBORN,
Da Costa Professor of Biology in Columbia College.
The first two volumes of this series were published in October; the third will be ready in April, and the fourth in October, 1895.
I. From the Greeks to Darwin.
Buckram. 8vo.
II. Amphioxus and the Ancestry of the Vertebrates.
Buckram. 8vo.
III. Fishes, Living and Fossil.
(In Preparation.)
IV. The Cell in Development and Inheritance.
(In Preparation.)
OPINIONS OF THE PRESS.
“The Columbia University of New York has decided to issue a series of small volumes on Biology, under the editorship of Dr. Osborn, the Da Costa Professor of Biology. It is not to be a library of elementary text-books in the ordinary sense of the term, but to consist chiefly of works dealing with certain fundamental problems which will concern students who have passed beyond preliminaries.”—Natural Science (London).
“If the other volumes in course of preparation by the professors in biology of Columbia University are up to the high standard of the present one (Amphioxus and the Ancestry of the Vertebrates), that institution is to be congratulated upon the enterprise of those who initiated the project.”—The American Naturalist (Philadelphia).
“The ‘Columbia University Biological Series’ is a new scientific publishing venture undertaken by Messrs. Macmillan & Co., under the general editorial supervision of Henry Fairfield Osborn. Numbers 1 and 2 of the series reach us, From the Greeks to Darwin, by H. F. Osborn, and Amphioxus and the Ancestry of the Vertebrates, by Arthur Willey. Judging from these volumes, the Columbia Biological Series will be of the utmost interest to students.”—Philadelphia Evening Telegram.
“The ‘Columbia University Biological Series,’ as inaugurated by the Macmillans, opens with a general review of the rise and progress of the evolution idea, from its first rudimentary perception by the Greek mind to the full development wrought out by Darwin.”—Milwaukee Sentinel.
MACMILLAN & CO., Publishers,
66 FIFTH AVENUE, NEW YORK.
[1] See especially Moseley, Nature, 1885, p. 417.
[2] Quart. Journ. Conch. i. p. 371.
[3] Manuel de Conchyliologie et de Paléontologie Conchyliologique. Dr. P. Fischer. Paris, 1887.
[4] κεφαλή, head; γαστήρ, stomach; σκάπτειν, to dig; πέλεκυς, an axe; πούς, ποδός, a foot.
[5] Also known as Lamellibranchiata, Conchifera, and Acephala.
[6] πτερόν, wing.
[7] γλῶσσα, tongue; φέρειν, to carry.
[8] λείπειν, to be wanting.
[9] ἀμφί, on both sides; νεὕρον, nerve, vessel. Some authorities regard the Amphineura as a distinct Order.
[10] πολύς, many; πλάξ, plate.
[11] πρόσω, in front. Often alluded to in the sequel as ‘operculate Gasteropoda.’
[12] κτενίδιον, a little comb.
[13] δὐω, two; mόnos, single; ὦτα, auricles; καρδία, heart.
[14] ὄπισθεν, behind.
[15] Pulmo, a lung.
[16] στὕλος, pillar; ὄμματα, eyes.
[17] The Ascoglossa are dealt with below (chap. xv.).
[18] Beudant, by very gradually changing the water, accustomed marine species to live in fresh, and fresh-water species to live in salt water.
[19] Braun, Arch. f. Naturk. Liv. (2), x. p. 102 f.
[20] Lindström, Oef. K. Vet. Förh. Stockh., 1855, p. 49.
[21] Mendthal, Schr. Ges. Königsb., xxx. p. 27.
[22] SB. K. Akad. Wiss. Wien, 1889, p. 4, but the view is not universally accepted.
[23] Not to Nassa, as has been generally held. The shape of the operculum, and particularly the teeth of the radula, show a much closer connexion with Cominella.
[24] E.g. Bouvier, Le Natural, 1889, p. 242.
[25] Köhler, Zool. Jahrb. vii. 1893, p. 1 f; Haller, Arb. Zool. Inst. Wien, x. p. 71.
[26] Plate, SB. kön. Preuss. Ak. Wiss. Berl. 1893. p. 959.
[27] E.g. Pelseneer, Bull. Sc. France Belg. xxiv. p. 347 f.
[28] E.g. Bergh, Zool. Jahrb. v. p. 1 f.
[29] Calkins, Amer. Nat. xi. p. 687.
[30] One step even further (or perhaps it should be termed a branch derivative) is seen in the genus Smaragdia, which is probably a Neritina which has resumed a purely marine habit of life.
[31] SB. Naturf. Gesell. Leipz. 1886–87, pp. 40–48.
[32] L. and F. W. Moll. of India, iv. p. 167.
[33] T. Scott, Journ. of Conch. v. p. 230.
[34] J. S. Gibbons, ibid. ii. p. 129.
[35] Bull. Soc. Linn. Nord, Abbeville, 1840, p. 150.
[36] Joly, Comptes Rendus, 1842, p. 460; compare W. A. Gain, Science Gossip, xxvii. p. 118.
[37] Von Martens, SB. Nat. Fr. Berl. 1881, p. 34.
[38] Moquin-Tandon, Moll. de France, i. p. 116.
[39] Journ. of Conch. iii. p. 321 f.; iv. p. 13; Science Goss. 1866, p. 158.
[40] Reichel, Zool. Anz. x. p. 488.
[41] Schumann, Schr. Ges. Danz. (2) vi. p. 159.
[42] Fischer and Crosse, Mexico, p. 437.
[43] Journ. de Conch. iv. p. 397, but the species observed is not mentioned.
[44] Bull. Mus. C. Z. Harv. iv. p. 378.
[45] W. Harte, Proc. Dubl. N. H. Soc. iv. p. 182.
[46] See on the whole subject of threads G. S. Tye, Journ. of Conch. i. p. 401.
[47] Zoologist, ii. p. 296; iii. p. 833; iv. p. 1216; iii. p. 1036; iv. p. 1216; iii. p. 1037.
[48] Ann. Nat. Hist. ii. 1838, p. 310.
[49] H. W. Kew, Naturalist, 1889, p. 103.
[50] Zeit. wiss. Zool. xlii. p. 203 f.
[51] Sci. Trans. R. Dubl. Soc. (2) iv. p. 520.
[52] Zoologist, iv. p. 1504; iii. p. 1038; iii. p. 943.
[53] H. W. Kew, l. c.
[54] Zoologist, xix. p. 7819.
[55] Naturalist, 1889, p. 55.
[56] H. W. Kew, l. c.
[57] W. G. Binney, Bull. Mus. C. Z. Harv. iv. p. 144.
[58] Naturalist, l. c.
[59] Science Gossip, 1885, p. 154.
[60] R. Standen, Journ. of Conch. vii. p. 197.
[61] Journ. of Conch. v. p. 43.
[62] A. Paladilhe in MS. letter.
[63] J. S. Gibbons, Quart. Journ. Conch. ii. p. 143.
[64] Bull. Mus. C. Z. Harv. iv. p. 193.
[65] l. c. p. 362.
[66] Animal Life, p. 59.
[67] Zoologist, 1861, p. 7400; Brit. Conch. i. p. 108.
[68] H. Ullyett, Science Gossip, xxii. (1886), p. 214.
[69] Descent of Man, i. p. 325, ed. 1.
[70] Amer. Nat. xv. 1881, p. 976.
[71] W. A. Gain, quoted by H. W. Kew in Naturalist, 1890, p. 307, an article to which I am much indebted.
[72] Ann. Mag. Nat. Hist. (5) xvi. p. 519.
[73] Science Gossip, 1882, pp. 237, 262.
[74] H. W. Kew, Naturalist, 1893, p. 149, another most valuable article.
[75] Garden, v. p. 201, quoted by Kew, ut sup.
[76] Kew, ut sup.
[77] Science Gossip, 1883, p. 163.
[78] T. D. A. Cockerell, Science Gossip, 1885, p. 211.
[79] Ann. Mag. Nat. Hist. (2) vi. (1850) p. 68.
[80] Ann. Mag. Nat. Hist. (2) vi. p. 489.
[81] Ibid. (3) iii. p. 448.
[82] Amer. Nat. xi. (1877) p. 100; Proc. Calif. Ac. iii. p. 329.
[83] Gaz. Med. Alger. 1865, 5th Jan. p. 9.
[84] Science Gossip, 1867, p. 40.
[85] Ann. Mag. Nat. Hist. (2) ix. p. 498.
[86] Journ. of Conch. vi. p. 101.
[87] Naturalist, 1889, p. 55.
[88] Malak. Blätt. (2) iv. pp. 43 and 221.
[89] Phil. Trans. 1854 (1856), p. 8.
[90] Naturalist, 1891, p. 75 f.; Conchologist, ii. 1892, p. 29.
[91] Taylor, Journ. of Conch. 1888, p. 299.
[92] See Tennent’s Ceylon, i. p. 221, ed. 5.
[93] W. A. Gain, Naturalist, 1889, p. 55; Brockmeier, Nachr. Deutsch. Malak. Gesell. xx. p. 113.
[94] Ann. Mag. Nat. Hist. (2) ix. p. 498.
[95] Journ. Conch. vii. 1893, p. 158 f.
[96] I succeeded in hatching out eggs of Helix aspersa, during the very warm summer of 1893, in 17 days.
[97] Nachr. Deutsch. Malak. Gesell. xx. p. 146.
[98] Raymond, Nautilus, iv. p. 6.
[99] Quoted by Oehlert, Rév. Sc. xxxviii. p. 701.
[100] Animal Life, Intern. Scientif. Ser. ed. 1, p. 395.
[101] Zoologist, 1886, p. 491.
[102] Thomas, quoted by Jeffreys, Brit. Conch. i. p. 30.
[103] Journ. of Conch. iv. p. 117.
[104] Rev. L. Jenyns, Observations in Nat. Hist. p. 318.
[105] Id. ib. p. 319.
[106] Further detailed examples will be found in Kew, The dispersal of Shells, pp. 5–26.
[107] P. Z. S. 1888, p. 358.
[108] W. A. Gain, Naturalist, 1889, p. 58.
[109] Das Wetter, Dec. 1892. Another case is recorded in Amer. Nat. iii. p. 556.
[110] Zoologist, x. p. 3430.
[111] Science Gossip, 1888, p. 281.
[112] Lecoq, Journ. de Conch. ii. p. 146.
[113] Bouchard-Chantereaux, Ann. Sci. Nat. Zool. (4) xvi. (1861) p. 197.
[114] Forel, Ann. Sci. Nat. (3) xx. p. 576; Bretonnière, Comptes Rendus, cvii. p. 566.
[115] Brit. Mus. Collection.
[116] Thomas, quoted by Récluz in Journ. de Conch. vii. 1858, p. 178.
[117] Nat. Hist. of Ceylon, p. 382. See also T. L. Taylor, Rep. Brit. Ass. for 1848, p. 82.
[118] Dr. R. E. Grant, Edinb. Phil. Journ. xiv. p. 188.
[119] Rep. Brit. Ass. for 1848, p. 80. The statement is confirmed by Rossmässler.
[120] Journ. of Conch. iv. p. 118.
[121] Zoologist, 1887, p. 29.
[122] Arch. Zool. Exp. Gén. (2) v. p. 459 f.
[123] Journ. of Conch. iii. p. 277; compare W. M. Webb, Zoologist, 1893, p. 281.
[124] Bull. Mus. Comp. Zool. Harv. iv. p. 85.
[125] Erjavec, Nachr. Deutsch. Malak. Gesell. 1885, p. 88.
[126] Crosse, Journ. de Conch. (3) xiv. (1874) p. 223.
[127] C. Wright, Zoologist, 1869, p. 1700.
[128] W. V. Legge, Zoologist, 1866, p. 190.
[129] Blackwall, Researches, p. 139.
[130] Barrow, Travels in South Africa, ii. p. 67.
[131] Loch Creran, p. 102.
[132] Cordeaux, Zoologist, 1873, p. 3396.
[133] Amer. Nat. xii. p. 695; Science Gossip, 1865, p. 79.
[134] Journ. Trent. N. H. Soc. 1887, p. 58.
[135] Ann. Nat. Hist. iii. 1893, pp. 238, 239.
[136] Rev. Nat. Sc. Ouest, 1891, p. 261.
[137] Petit de la Saussaye, Journ. de Conch. iii. p. 97 f.
[138] J. W. Williams, Science Gossip, 1889, p. 280.
[139] Noack, Zool. JB. ii. p. 254.
[140] La Nature, xv. (2) p. 46.
[141] François, Arch. Zool. Exp. Gén. (2) ix. p. 240.
[142] A. Lang, Ber. Naturf. Ges. Freib. vi. 1892, p. 81.
[143] A. P. Thomas, Q. J. Micr. Sc. N. S. xxiii. (1883) p. 99.
[144] H. Woodward, P. Z. S. 1886, p. 176.
[145] W. E. Collinge, Zoologist, 1890, p. 467.
[146] Proc. Linn. Soc. N. S. Wales, ix. p. 944.
[147] Zoologist, xviii. (1860) p. 7136.
[148] A. Adams, Samarang, vol. ii. Zoology, p. 357.
[149] In Thomson’s British New Guinea, p. 283.
[150] Animal Life, p. 395. It should be mentioned that Von Möllendorff (Ber. Senck. Ges. 1890, p. 198) ridicules the whole theory.
[151] Von Martens, SB. Nat. Fr. Berl. 1891, p. 83.
[152] Von Martens, ibid. 1887, p. 183.
[153] SB. Nat. Gesell. Leipz. xiii.-xiv. p. 45.
[154] Garstang, Journ. Mar. Biol. Ass. N. S. i. p. 432; Giard, Bull. Sci. Fr. Belg. 1888, p. 502 f.
[155] Nautilus, vi. 1892, p. 90.
[156] R. F. Scharff, Sci. Trans. R. Dubl. Soc. (2) iv. p. 553 f.
[157] Q. Journ. Micr. Sci. N. S. xxxi. (1890) p. 41 f.
[158] A detailed account is given in Proc. Liverp. Biol. Soc. iv. (1890) pp. 150–163.
[159] Journ. Mar. Biol. Ass. N. S. i. p. 418 f.
[160] Garstang, Conchologist, ii. p. 49.
[161] Hecht, Comptes Rendus, cxv. p. 746.
[162] Conchologist, ii. p. 130.
[163] Described as a Cypraea, but no doubt an Ovula or Pedicularia: CB. Bakt. Par. v. p. 543.
[164] Von Graff, Z. wiss. Zool xxv. p. 124.
[165] Proc. Amer. Phil. Soc. xxv. p. 231.
[166] Ergeb. naturw. Forsch. Ceylon, abstr. in Journ. Roy. Micr. Soc. (2) vi. p. 412.
[167] Voyage of the Samarang, Moll. p. 69, Pl. xi. f. 1; p. 47, Pl. xvii. f. 5.
[168] E. A. Smith, Ann. Mag. Nat. Hist. (6) iii. p. 270.
[169] Journ. de Conch. (3) xxix. p. 101.
[170] Zool. Jahrb. Abth. f. Syst. v. p. 619.
[171] See especially Semper, Animal Life, Ed. 1, p. 351.
[172] Gould, Moll. of U.S. expl. exped. 1852, p. 207 (St. acicula, from Fiji).
[173] Stimpson, Proc. Bost. Soc. N. H. vi. 1858, p. 308.
[174] Pidgeon, Nature, xxxix. p. 127.
[175] W. Anderson Smith, Loch Creran, p. 46.
[176] Smart, Journal of Conch. v. p. 152.
[177] Animal Life, p. 351.
[178] Journ. of Conch. vi. 1891, p. 399.
[179] Ann. Mag. N. H. (6) vii. p. 276.
[180] Stimpson, quoted by Jeffrey’s Brit. Conch. ii. 194.
[181] Stimpson, Journ. Bost. Soc. N. H. vi. 1857, p. 48.
[182] E. H. Matthews, Conchologist, ii. p. 144.
[183] Thus Limnaea involuta, which is almost universally regarded as a good and distinct species, has been held to be no more than a variety of L. peregra produced by locality; see Zoologist, 1889, p. 154.
[184] J. W. Taylor, Journ. of Conch. v. p. 289, an interesting article, with many useful references.
[185] Möbius, Report on ‘Pommerania’ Exped. pp. 138–141.
[186] Journ. de Conchyl. xxiii. 1875, p. 105.
[187] J. W. Taylor ut sup. p. 300.
[188] Sci. Trans. R. Dubl. Soc. (2) iv. p. 555.
[189] J. S. Gibbons, Journ. of Conch. ii. p. 129.
[190] C. H. Morris, ibid. vii. p. 191.
[191] F. M. Hele, ibid. iv. p. 93.
[192] T. D. A. Cockerell, Science Gossip, 1887, p. 67.
[193] J. G. Jeffreys, British Conchology, vol. i. p. 214.
[194] Journ. of Conch. vi. p. 123.
[195] Phil. Trans. 1889, vol. 180 B, p. 207. A somewhat similar case (the celebrated Steinheim series of Planorbis) is dealt with by Hilgendorf, MB. Akad. Berl. 1866, p. 474; and Hyatt, Proc. Amer. Ass. Sc. xxix. p. 527.
[196] J. B. Bridgman, Quart. Journ. Conch. i. p. 70.
[197] W. C. Hey, Journ. of Conch. iii. p. 268.
[198] Zool. Anz. xiii. p. 662.
[199] J. Madison, Journ. of Conch. v. p. 260.
[200] Quart. Journ. Conch. i. 339.
[201] Whitfield, Bull. Amer. Mus. N. H. i. p. 29.
[202] Amer. Nat. xiv. p. 51.
[203] Animal Life, Ed. 1, p. 160 f.
[204] Conch. Syst. ii. p. 262 n.
[205] P. L. Simmonds, Commercial Products of the Sea, p. 278.
[206] Benderloch, p. 118.
[207] C. Hedley in J. P. Thomson, Brit. New Guinea, p. 283.
[208] Most of the above facts are derived from a study of a collection of native implements, weapons, ornaments, etc., in the Antiquarian Museum at Cambridge.
[209] Thurston, Notes on the Pearl and Chank Fisheries, Madras, 1890.
[210] See in particular, P. L. Simmonds, The Commercial Products of the Sea.
[211] H. Friend, Field Club, iv. 1893, p. 100.
[212] Nature, xxxi. 1885, p. 492.
[213] W. Anderson Smith, Benderloch, p. 173.
[214] Dominique, Feuill. Nat. xviii. p. 22.
[215] SB. Nat. Fr. Berl. 1889, p. 197.
[216] A. Adams, Voyage of the ‘Samarang,’ ii. p. 308.
[217] Much information has been derived, on this subject, from Bertram’s Harvest of the Sea, Simmonds’ Commercial Products of the Sea, the publications of the Fisheries Exhibition, especially vol. xi. (Anson and Willett); see also Philpots, Oysters and all about them.
[218] Juvenal, Sat. iv. 140–142.
[219] Hist. Nat. ix. 79.
[220] Vol. Max. ix. 1.
[221] Quart. Journ. Micr. Sc. xxvi. p. 71.
[222] See G. H. Lewes, Sea-side Studies, p. 339.
[223] Bull. U.S. Fish. Comm. v. p. 161.
[224] W. Anderson Smith, Loch Creran, p. 228.
[225] Longmans’ Magazine, June 1889.
[226] St. James’s Gazette, 6th January 1893.
[227] Also at Arcachon (W. A. Herdman, Nature, 1893, p. 269).
[228] See especially Hoek, Tijdschr. Ned. Dierk. Vereen, Suppl. Deel, i. 1883.
[229] Benderloch, p. 136.
[230] This is the view of E. Ray Lankester, Quart. Journ. Micr. Sc. xxvi. 80.
[231] De Quatrefages, Rambles of a Naturalist.
[232] Quoted by Jeffreys, Brit. Conch., ii. p. 109.
[233] M. S. Lovell, Edible Mollusks, p. 49.
[234] Science, vii. p. 175.
[235] Hist. Nat. ix. 82.
[236] De re rustica, iii. 14.
[237] Epistles, i. 15.
[238] Hor. Sat. II., iv. 58, tr. Conington.
[239] Roberts, Zoologist, 1885, p. 425.
[240] Hist. Nat. xxx. 15, 19.
[241] Science Gossip, 1891, p. 166.
[242] Jeffreys, Brit. Conch. iii. p. 355.
[243] W. Clark, Mag. Nat. Hist. xvi. p. 466.
[244] Examples will be found in Journ. Linn. Soc. Zool. xi. p. 90; Ann. Sc. Nat. xx. p. 472; Zeit. wiss. Zool. xxiv. p. 419.
[245] Herdman, Proc. Liverp. Biol. Soc. iii. p. 30.
[246] Garrett, Journ. Ac. Nat. Sc. Phil. viii. (1880).
[247] J. Bladon, Zoologist, xvi. p. 6272.
[248] Lo Bianco, MT. Zool. Stat. Neap. viii. p. 414.
[249] Animal Life, pp. 126, 135.
[250] R. Rimmer, Land and Fresh-Water Shells, p. 119.
[251] Journ. de Conch. ii. p. 245.
[252] Journ. de Conchyl. iii. p. 107.
[253] Jeffreys, Brit. Conch. iii. p. 359; Sauvage, Journ. de Conchyl. xxi. p. 122.
[254] Hermaphroditism seems to occur in (a) whole families, e.g. Anatinidae and the Septibranchia; (b) genera, e.g. Cyclas, Pisidium; (c) single species, e.g. in the generally dioecious genera Ostrea, Pecten, Cardium.
[255] δὐω, two; μόνος, single; γόνος, semen; πόρος, passage.
[256] Von Brunn, Arch. Mikr. Anat. xxiii. p. 413.
[257] Hist. Anim. v. 6 and 12, iv. 1, ed. Bekker, 1837.
[258] ‘On pourra constater si ce ne seraient pas des parties détachées de quelque céphalopode dans le but de servir à le fécondation,’ Hist. Nat. Helminthes, 1845, p. 482.
[259] Steenstrup, Ann. Mag. Nat. Hist. (2), xx. p. 81 f.
[260] C. Ashford, Journ. of Conch. iii. p. 239, iv. pp. 69, 108.
[261] W. E. Collinge, Zoologist, 1890, p. 276.
[262] Pelseneer, Comptes Rendus, cx. p. 1081.
[263] Kon. Vet. Akad. Handl. 1848, pp. 329–435.
[264] P. Z. S. 1891, p. 52 f.
[265] The result of some experiments by Professor Herdman upon Littorina rudis, tends to show that it can live much better in air than in water, and goes far to support the view that the species may be undergoing, as we know many species must have undergone (see p. 20), a transition from a marine to a terrestrial life. It was found that marked specimens upon the rocks did not move their position for thirty-one successive days (Proc. Liverp. Biol. Soc. iv. 1890, p. 50).
[266] Diminutive of κτείς, a comb.
[267] Stoliezka, quoted in Journ. de Conch. xviii. p. 452.
[268] ζύγος, a yoke, from the symmetrical position of the branchiae.
[269] Pelseneer, ‘Challenger’ Reports, vol. xxiii. part lxvi.
[270] Zoologist, xii. p. 4248.
[271] Mollusques de France, i. p. 81.
[272] N. Denk. Schw. Ges. xxix. (2) p. 196 f.
[273] Bergh, Morph. Jahrb. x. p. 172.
[274] P. Fischer, Journ. de Conch. ix. p. 101.
[275] Bull. Mus. C. Z. Harv. xviii. p. 434.
[276] Pelseneer, Comptes Rendus, cvi. p. 1029.
[277] E.g. Kollmann, Zeit. wiss. Zool. xxvi. p. 87.
[278] Proc. Roy. Soc. 1873, p. 70.
[279] Griesbach (Arch. mikr. Anat. xxxvii. p. 22) finds haemoglobin in several bivalves, e.g. Poromya granulata, Tellinata planata, Arca Noae, and Pectunculus glycimeris.
[280] Trans. Roy. Soc. N. S. Wales, xxii. p. 106.
[281] Pelseneer, Comptes Rendus, cx. p. 154.
[282] Science, iv. p. 50.
[283] P. Fischer, Journ. de Conchyl. (3) xxvii. p. 201.
[284] Journ. of Conch. vi. p. 349 ff.
[285] Quart. Journ. Micr. Sc. N.S. xv. p. 37.
[286] Ann. Mag. Nat. Hist. (2), xx. p. 336.
[287] V. Willem (Arch. Biol. ut infr.) denies this, and declares that Cyclostoma is only very sensitive to movements. The present writer has often approached, with the greatest care, a crawling Cyclostoma, but it always withdrew into its shell or fell to the ground when approached within about 10 or 12 inches.
[288] Arch. Biol. xii. 1892, p. 57.
[289] ‘Challenger’ Reports, Zoology, vol. xxvii. part lxxiv. p. 3.
[290] Animal Life, p. 372 f.
[291] Bergh, Morph. Jahrb. x. p. 172.
[292] Ann. Mag. Nat. Hist. (5) xiv. p. 141.
[293] The nature of the grouping of the eyes into rows varies considerably in different species. As a rule, the rows radiate from the beak, but occasionally they run parallel to the girdle. In Tonicia lineolata Fremb., they are grouped, as it were, under the shelter of strongly marked longitudinal wavy lines.
[294] Shell-Eyes in other Mollusca.—The Rev. J. E. Tenison-Woods (Trans. Linn. Soc. N. S. Wales, xxii. p. 106) is of opinion that ‘shell-eyes’ are by no means confined to the Chitonidae, but that, in fact, multiplicity of eyes of this kind is the rule rather than the exception among the Mollusca. He finds (1) exceedingly minute and numerous ‘eyes’ on the outer surface of the shell in both univalves and bivalves; (2) large and solitary ‘eyes’ in the shell substance; (3) eyes on the mantle lobes in both univalves and bivalves; (4) eyes on the opercula.
[295] Mitth. Stat. Zool. Neap. v. p. 447 ff.
[296] W. Patten, Mitth. Zool. Stat. Neap. vi. (1886) pp. 546, 605 f.
[297] Benderloch, p. 136.
[298] Quart. Journ. Micr. Soc. xx. p. 443.
[299] Quart. Journ. of Conch. i. p. 368.
[300] British Conchology, i. p. xxviii.
[301] Science Gossip, 1865, p. 259.
[302] Mollusques de France, i. p. 130.
[303] E.g. Sochaczewer, Zeits. wiss. Zool. xxxv. p. 30.
[304] Zool. Anz. 1882, p. 472.
[305] Zoologist, iv. p. 1266.
[306] Journ. Mar. Biol. Ass. N.S. i. p. 217.
[307] Moquin-Tandon, Moll. de France, i. p. 133.
[308] Zool. Jahrb. Anat. iv. (1890) p. 501.
[309] Baudon, Rév. Mag. Zool. 1852, p. 575.
[310] Arch. Zool. Exp. Gén. (2) v. 1887, p. 2; compare also C. H. Hurst, Natural Science, ii. pp. 360, 421.
[311] Compare Pelseneer, Bull. Sci. Fr. Belg. (3) xix. pp. 107, 182.
[312] Pelseneer, Arch. Biol. viii. p. 723.
[313] Also known as labial and supra-oesophageal ganglia.
[314] Wivén, however (K. Sv. Vet. Ak. Handl. xxiv. 1892, No. 12), describes transverse connectives in Chaetoderma.
[315] στρεπτός, twisted; εὐθύς, straight.
[316] With the exception of Actaeon, which is streptoneurous (Bouvier, Comptes Rendus, cxvi. p. 68).
[317] This fusion of the cerebral and pleural ganglia and the consequent union of the cerebro-pedal and pleuro-pedal commissures can be recognised by sections of the mass (Pelseneer, Comptes Rendus, cxi. p. 245).
[318] There is practically no pharynx in the Pelecypoda, the mouth opening directly into the oesophagus.
[319] Radere, to scrape; ὸδούς, tooth; φέρειν, to carry.
[320] The mechanism of the radula has been dealt with by Geddes, Trans. Zool. Soc. x. p. 485. Rücker has observed (Ber. Oberhess. Gesell. Nat. Heilk. xxii. p. 207) that the radula in Helix pomatia is the product of five rows of cells; the use of the first row is uncertain, the second forms the membrane of the radula, while rows three to five originate the teeth.
[321] Jahrb. Deut. Malak. Gesell. iii. p. 193.
[322] The whole of the radulae and jaws figured in this work are taken from the original specimens in the collection of the Rev. Prof. H. M. Gwatkin, who has always been ready to give me the run of his cabinets, which probably contain the finest series of radulae in the world. To his kindness I owe the following description of the process of mounting: “The first step is to obtain the radula. Dissection is easy in species of a reasonable size. On opening the head from above, so as to lay open the floor of the mouth, the radula itself is seen in most of the marine species, though in others it is contained in a sort of proboscis; and in the Pulmonata and others the student will find the buccal mass, with commonly a brown mandible at its front end, and the lingual ribbon in its hinder part. The teeth may be recognised by their silvery whiteness, except in a few cases like Patella and Chiton, where they are of a deep brown colour. When obtained, the radula may be cleaned by boiling in a solution of caustic potash. There is no risk of injury if the solution is not too strong.
“Smaller species may be treated more summarily. The proboscis, the buccal mass, or even the whole animal may be thrown into the potash solution and boiled till scarcely anything is left but the cleaned radula. Remains of animals dried inside the shell may be similarly dealt with, after soaking in clean water. With a little care, this process will answer for shells down to the size of Ancylus or Rissoa. The very smallest (Carychium, Tornatellina, Skenea, etc.) must be crushed on the slide and boiled on it, after removing as much as possible of the broken shell. The radula can then be searched for under the microscope, and washed and mounted on the slide.
“The student must be warned that though the general process is simple, there are difficulties in particular cases. In the Pulmonata, for example, membranes on both sides of the radula need careful removal. Murex, Purpura, and most of the Taenioglossa have the side teeth folded down over the central, so that the arrangement is not well seen till they have been brushed back. The Cones, again, have no basal membrane at all, so that if the potash is not used with great care, the single teeth will fall asunder and be lost. Perhaps the worst case is where a large animal has a radula as small as that of a Rissoa, like Turritella, Harpa, or Struthiolaria, or where the radula is almost filmy in its transparency, like those of Actaeon and the small Scalaria.
“When once the radula is laid out, the mounting is commonly easy. Canada balsam makes it too transparent. Fluids may be used, and are almost necessary for thick radulae like those of large Chitons; but the best general medium is glycerine jelly. It runs under the cover glass by capillary attraction, and may be boiled (though only for a moment) to get rid of air bubbles. It should then be left unfinished for several weeks. If cracks appear, the reason is either that the jelly is a bad sample, or that it has been boiled too long, or (commonly) that the object is too thick; and there is not often any difficulty in remounting. I have no serious complaint of want of permanence against the medium, if I may speak from a pretty wide experience during the last twenty years.”
[323] The substance both of the jaw and radula is neither crystalline nor cellular, but laminated. Chitin is the substance which forms the ligament in bivalves, the ‘pen’ in certain Cephalopoda, and the operculum in many univalves. Neither silica nor keratine enter into the composition of the radula.
[324] τόξον, arrow; ῥάχις, ridge, sharp edge; ταινία, ribbon; πτηνός, winged; γυμνὀς, bare; ῥιπίς, fan; δοκός, beam.
[325] V. concinna, according to Schacko (Conch. Mitth. i. p. 126, Pl. xxiv. f. 5); the lateral is large, strong, unicuspid on a broad base.
[326] In some cases (e.g. Hyalinia inornata) the laterals are very few, while in Zonites laevigatus the first side tooth is more of a marginal than a lateral.
[327] Semon, Biol. Centralbl. ix. p. 80.
[328] According to Moquin-Tandon (Moll. de France, i. p. 44) this process in Bithynia is attached by one end to the wall of the stomach. Vivipara, with two jaw pieces, does not possess this stylet; Bithynia, which does possess it, has no jaw.
[329] J. H. Vanstone, Journ. Linn. Soc. xxiv. p. 369.
[330] Biol. Centralbl. vii. p. 683; SB. Ges. Nat. Fr. 1890, p. 42; Mag. Nat. Hist. (2) v. 1850, p. 14.
[331] νεφρός, kidney.
[332] Ann. Mag. Nat. Hist. (2) xvi. p. 298.
[333] See, for instance, Quart. Journ. Conch. i. p. 340 (Cyl. Raveni): Jahrb. Deut. Malak. Gesell. 1879, p. 98 (Clausilia dubia).
[334] Cailliaud, Journ. de Conchyl. vii. p. 231; Gassies, ibid. p. 44.
[335] Arch. Naturgesch. xlii. p. 209.
[336] Dr. W. B. Carpenter, Rep. Brit. Ass. xiii. p. 71; xiv. p. 1; xvii. p. 93; J. S. Bowerbank, Trans. Micr. Soc. i. p. 123; Ehrenbaum, Zeit. wiss. Zool. xli. p. 1.
[337] See also p. 258.
[338] J. E. Gray, Phil. Trans. 1833, p. 774 f.
[339] J. E. Gray, Phil. Trans. 1833, p. 774 f.
[340] Journ. de Conchyl. iv. p. 424.
[341] Journ. de Conchyl. xii. p. 3.
[342] T. Scott, Journ. of Conch., 1887, p. 230.
[343] M. de Villepoix, Comptes Rendus, cxiii. p. 317.
[344] Proc. Ac. Nat. Sc. Phil., 1892, p. 350.
[345] Mr. B. B. Woodward has recently pointed out (P. Z. S. 1892, p. 528) a very remarkable method of shell absorption and growth in Velates and certain other Neritidae.
[346] The only exception appears to be Pedipes, while in Cassidula and Scarabus the absorption is partial (Crosse and Fischer, Journ. de Conch. xxx. p. 177 f.).
[347] Strombus and Pteroceras (see Fig. 99, p. 200) exceptionally develop a siphonal notch which is distinct from the anterior canal.
[348] The columella, as distinct from the columella lip, is the solid pillar of shell round which the whorls are coiled (Fig. 177), the lower, or anterior portion of which alone is usually visible.
[349] J. E. Gray, Phil. Trans. 1833, p. 812.
[350] W. H. Dall, Amer. Journ. Sc. xxxviii. p. 445 f.
[351] The term epidermis, as distinct from periostracum, is properly restricted to the outer layer of the skin of the mantle and body generally.
[352] J. Lewis, Proc. Bost. Soc. vi. p. 149.
[353] Journ. of Conch. v. p. 66.
[354] The Dispersal of Shells, pp. 182–195.
[355] E. A. Smith, P. Z. S. 1892, p. 259.
[356] C. T. Musson, Proc. Linn. Soc. N. S. Wales (2), v. p. 883.
[357] Scient. Results Sec. Yarkand Exped. “Mollusca,” pp. 1–16.
[358] Mr. H. W. Kew, The Dispersal of Shells, has brought together a very large series.
[359] The Naturalist in Nicaragua, p. 334 f.
[360] Morelet, Journal de Conch. 1875, p. 194.
[361] Pollonera, Boll. Mus. Zool. Torino, v. 1890, No. 87.
[362] South and south-western France, however, belong to the Mediterranean Sub-region.
[363] The coast-line of north-east China, including Corea and Japan to north Niphon, is much more definitely tropical than the adjacent inland districts. The coast-line, therefore, must be placed in the Oriental Region, while the inland districts belong to the Palaearctic Region.
[364] Biol. Centralbl. ii. p. 208.
[365] Craven, Journ. de Conchyl. (3) xxviii. p. 101.
[366] Jahrb. Deutsch. Malak. Gesell. viii. p. 278.
[367] Netchayeff, Kazan Soc. Nat. xvii. fasc. 5.
[368] Fauna der Congerien-Schichten, p. 142.
[369] Streptaxis is a remarkable instance of a mainland genus. Although abundant in the Oriental, Ethiopian, and Neotropical regions, it never seems to occur on any of the adjacent islands, except in the case of Trinidad (1 sp.), which is practically mainland. Omphalotropis, on the other hand, is the exact reverse of Streptaxis in this respect, occurring all over Polynesia and the Malay Is., as far west as Borneo, as well as on the Mascarenes, but never, save in a doubtful case from China, on the mainland of Asia, Australia, or Africa.
[370] The Amboyna group has been much the better explored. Common to both groups are one sp. each of Kaliella, Trochomorpha, Opeas, Leptopoma, Cyclotus, Helicina.
[371] A. H. Cooke, P. Z. S. 1892, pp. 447–469.
[372] Mysol, with 2 Chloritis, 1 Insularia, 1 Cristigibba, is decidedly Papuan.
[373] See especially C. Hedley, Note on the Relation of the Land Mollusca of Tasmania and New Zealand, Ann. Mag. Nat. Hist. (6) xiii. p. 442.
[374] Hedley and Suter, Proc. Linn. Soc. N. S. Wales (2), vii. p. 613. Twenty-one species are “introduced.”
[375] Nine species have been introduced: 6 from Europe, 2 from the West Indies, 1 from the Western Isles.
[376] It is by no means implied that unbroken land communication between India and Madagascar, across the Indian Ocean, ever existed. A series of great islands, whose remains are attested by the Chagos and other banks, would be quite sufficient to account for the results, as we find them. See especially Medlicott and Blanford, Geology of India, vol. i. p. lxviii.
[377] Journ. Cinc. Soc. Nat. Hist. iii. p. 317. The number is doubtless susceptible of very considerable reduction, say by one-half at least.
[378] Simpson, Amer. Nat. xxvii. 1893, p. 354.
[379] Compare von Martens, Malak. Blätt. 1868, p. 169; von Ihering, Nachr. Deutsch. Malak. Gesell. 1891, p. 93.
[380] The distribution of some Pteropoda has been worked out by Munthe, Bih. Svensk. Ak. Handl. XII. iv. 2, by Pelseneer “Challenger” Rep., Zool. xxiii., and by Boas, Spolia Atlantica.
[381] Bull. Mus. C. Z. Harv. xiv. p. 202; xxiii. p. 34 f.
[382] See papers in P. Z. S. 1878–85.
[383] A break in this uniformity may be found underneath the course of a great oceanic current like the Gulf Stream, which rains upon the bottom a large amount of food. A. Agassiz (Bull. Mus. C. Z. Harv. xxi. p. 185 f.) explains in this way the richness of the fauna of the Gulf of Mexico as compared with that of the west coast of tropical America.
[384] On the western coasts of Europe and America, where the change in surface temperature is very gradual, Purpura lapillus (the west American ‘species’ are at best only derivatives) is able to creep as far south as lat. 32° (Mogador) in the former case, and lat. 24° (Margarita Bay) in the latter, the mean annual temperature of the surface water being 66° off Mogador, with an extreme range of only 8°, and that of Margarita Bay 73°, with an extreme range of only 5°. On the eastern coasts, where the Pacific and Atlantic gulf-streams cause a sudden change of temperature, the Purpura is barred back at points many degrees farther north, viz. at lat. 41° (Hakodadi), surface temperature 52°, extreme range 25°; and at lat. 42° (Newhaven), surface temperature 52°, extreme range 30°.
[385] E. A. Smith, P. Z. S. 1890, pp. 247, 317.
[386] A. H. Cook, Ann. Mag. Nat. Hist. (5) xviii. (1886) p. 380 f; E. A. Smith, P. Z. S. 1891, p. 391 f.
[387] C. Keller, Neue denksch. Schw. Gesell. xxviii. 1883, pt. 3.
[388] According to Tate (Trans. Roy. Soc. S. Austr. 1887–88, p. 70), ‘Australian’ species predominate at Freemantle (32°), but Tenison-Woods (J. Roy. Soc. N. S. Wales, xxii. p. 106) holds that the tropical fauna extends as far south as Cape Leeuwin (34°), and that the Australian forms are not predominant until the extreme south. Tenison-Woods regards Cape Byron (31°) as the limit of the tropical fauna on the east coast, while some characteristic tropical genera reach Port Jackson, and a few (e.g. Cypraea annulus) Tasmania.
[389] A full account of the distribution of Voluta is given by Crosse, Journ. de Conchyl. (3) xix. p. 263.
[390] Usually known as ‘Patagonian,’ but since the Magellanic Sub-region includes a considerable part of Patagonia, and since the greater part of sub-region (6) lies out of Patagonia, it has been thought advisable to change the name.
[391] Amer. Nat. xx. p. 931.
[392] W. H. Dall, Proc. Biol. Soc. Washington, v. p. 1 f.
[393] Trans. Connect. Acad. v. p. 177; Zoologist, 1875, p. 4502.
[394] Rep. Scotch Fish. iii. 1885, App. F, p. 67.
[395] Nautilus, vi. 1892, p. 82.
[396] Journ. Mar. Zool. i. pp. 3, 9.
[397] Rep. Brit. Assoc. 1844, Transactions, p. 74; P. Z. S. 1839, p. 35.
[398] It is convenient, but not morphologically correct, to apply the terms ‘ventral’ and ‘dorsal’ in this sense.
[399] φραγμός, partition; σήπιον, cuttle-bone; χόνδρος, long cartilage.
[400] μυέω, close the eyes; ὕψις, sight; contrasted with Oigopsidae (οἰγω, open).
[401] The classification is that of Foord, Catal. Fossil Cephal. Brit. Mus., 1888.
[402] Saville Kent, Proc. Roy. Soc. Queensland, vi. p. 229.
[403] J. Power, Ann. Mag. N. H. (2) xx. p. 334; P. Z. S. 1836, p. 113; Arch. Zool. Exp. Gén. (3) i. 1893, p. 105.
[404] In deference to Bergh’s high authority, the position of a sub-order is here given to the Ascoglossa. It may be doubted whether that position will stand the test of further investigation, and whether the families concerned will not be added to the Cladohepatic Nudibranchs.
[405] This family has also been classified with the Bulloidea and with the Aplysioidea.
[406] It appears more convenient to treat the whole group together, rather than deal with the two sections separately.
[407] An operculum is said to exist in the young forms of Auricula and Parmacella.
[408] Proc. Ac. Philad. 1892, p. 390.
[409] Compare Jackson, Amer. Nat. xxv. p. 11 f.
[410] “A Monograph of the British Fossil Brachiopoda,” Palaeontographical Society, London, vols. i.-v. 1851–84.
[411] Ibid. vol. vi. 1886.
[412] “Contributions to the Anatomy of the Brachiopoda,” Proc. Roy. Soc., vol. vii.
[413] “Untersuchungen über den anatomischen u. histologischen Bau der Brachiopoda Testicardinia,” Jenaische Zeitschrift, vol. xvi., 1883.
[414] “On a living Spinose Rhynchonella from Japan,” Ann. Mag. Nat. Hist., 5th ser., vol. xvii., 1886
[415] Loc. cit. p. 465.
[416] Shipley, “On the Structure and Development of Argiope,” Mitt. aus d. Zool. Stat. zu Neap. Bd. iv. 1883.
[417] Schulgin, “Argiope Kowalevskii,” Zeit. f. wiss. Zool. Bd. 41, 1885.
[418] American Jour. of Sci. and Arts, 3rd series, vol. xvii. 1879.
[419] Loc. cit. p. 470.
[420] “Recherches sur l’Anat. des Brachiopodes Inarticules,” Arch. Zool. Exp. (2), Tome iv., 1886.
[421] “Untersuchungen über den Bau der Brachiopoden,” Jena, 1892.
[422] “Vorläufige Mittheilungen über Brachiopoden,” Zool. Anz. Bd. viii. 1885.
[423] Hancock’s nomenclature is here used. The corresponding names used by King and Brooks are placed in brackets. Their nomenclature is used by many palaeontologists, and is adopted in Fig. 322.
[424] Development of the Brachiopoda, 1873 (Russian).
[425] “Histoire de la Thécidie,” Ann. d. Sci. Nat., Sér. 4, vol. xv., 1861.
[426] “On the Early Stages of Terebratulina septentrionalis,” Mem. Boston Soc. Nat. Hist., vol. ii., 1869. “On the Development of Terebratulina,” Ibid. vol. iii., 1873.
[427] “Choses de Nouméa,” Arch. d. Zool. exp. et gen., 2nd ser., vol. ix., 1891.
[428] J. Barrande, Syst. Silur. Bohème, vol. v., 1879. Hall and Clarke, Introd. Palaeozoic. Brach. (Palaeont. of New York, 1892–1894). Davidson, Monogr. Brit. Foss. Brach. (Palaeont. Soc., 1851–1884). Waagen, Salt Range Fossils (Mem. Geol. Surv. India, 1879–1885).
[429] The results of the investigations of King (Ann. Mag. Nat. Hist., 4th ser., vol. xii., 1873) and of Brooks (Chesapeake Zool. Laboratory, Scientific Results, p. 35, 1879), and the simple nomenclature of these authors are here followed in preference to those of others, owing to the difference of opinion amongst anatomists of the functions and homologies of the muscles. The lateral muscles enable the valves to move backwards and forwards on each other; the centrals close the shell; the umbonals open it; and the transmedians allow a sliding sideways movement of one valve across the other (see also p. 477).
[430] Davidson and King, Quart. Jour. Geol. Soc., xxx. (1874), p. 124.
[431] Amer. Jour. Science, 1890–1893.
Transcriber’s Notes:
1. Obvious printers’, punctuation and spelling errors have been
corrected silently.
2. Where hyphenation is in doubt, it has been retained as in the
original.
3. Some hyphenated and non-hyphenated versions of the same words have
been retained as in the original.